Impaired macrophage phagocytosis of bacteria in severe asthma
- PMID: 24972601
- PMCID: PMC4086996
- DOI: 10.1186/1465-9921-15-72
Impaired macrophage phagocytosis of bacteria in severe asthma
Abstract
Background: Bacteria are frequently cultured from sputum samples of severe asthma patients suggesting a defect in bacterial clearance from the airway. We measured the capacity of macrophages from patients with asthma to phagocytose bacteria.
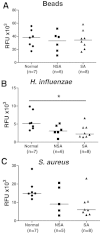
Methods: Phagocytosis of fluorescently-labelled polystyrene beads, Haemophilus influenzae or Staphylococcus aureus by broncholaveolar lavage alveolar macrophages (AM) and by monocyte-derived macrophages (MDM) from non-asthmatics, mild-moderate and severe asthmatic patients was assessed using fluorimetry.
Results: There were no differences in phagocytosis of polystyrene beads by AMs or MDMs from any of the subject groups. There was reduced phagocytosis of Haemophilus influenzae and Staphylococcus aureus in MDMs from patients with severe asthma compared to non-severe asthma (p < 0.05 and p < 0.01, respectively) and healthy subjects (p < 0.01and p < 0.001, respectively). Phagocytosis of Haemophilus influenzae and Staphylococcus aureus by AM was also reduced in severe asthma compared to normal subjects (p < 0.05). Dexamethasone and formoterol did not suppress phagocytosis of bacteria by MDMs from any of the groups.
Conclusions: Persistence of bacteria in the lower airways may result partly from a reduced phagocytic capacity of macrophages for bacteria. This may contribute to increased exacerbations, airway colonization and persistence of inflammation.
Figures





References
-
- Chung KF, Godard P, Adelroth E, Ayres J, Barnes N, Barnes P, Bel E, Burney P, Chanez P, Connett G, Corrigan C, de Blic J, Fabbri L, Holgate ST, Ind P, Joos G, Kerstjens H, Leuenberger P, Lofdahl C-G, McKenzie S, Magnussen H, Postman, Saetta D, Salmeron S, Silverman M, Sterk P. Difficult/therapy-resistant asthma: the need for an integrated approach to define clinical phenotypes, evaluate risk factors, understand pathophysiology and find novel therapies. ERS Task Force on Difficult/Therapy-Resistant Asthma. European Respiratory Society. Eur Respir J. 1999;13:1198–1208. - PubMed
-
- Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, Calhoun WJ, Castro M, Chung KF, Clark MP, Dweik RA, Fitzpatrick AM, Gaston B, Hew M, Hussain I, Jarjour NN, Israel E, Levy BD, Murphy JR, Peters SP, Teague WG, Meyers DA, Busse WW, Wenzel SE. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. - PMC - PubMed
-
- Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bønnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, Stage M, Pipper CB. Childhood asthma after bacterial colonization of the airway in neonates. N Eng J Med. 2007;357:1487–1495. - PubMed
-
- Martin RJ, Kraft M, CHU HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

