RGS5 promotes arterial growth during arteriogenesis
- PMID: 24972930
- PMCID: PMC4154134
- DOI: 10.15252/emmm.201403864
RGS5 promotes arterial growth during arteriogenesis
Abstract
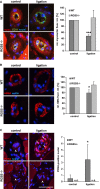
Arteriogenesis-the growth of collateral arterioles-partially compensates for the progressive occlusion of large conductance arteries as it may occur as a consequence of coronary, cerebral or peripheral artery disease. Despite being clinically highly relevant, mechanisms driving this process remain elusive. In this context, our study revealed that abundance of regulator of G-protein signalling 5 (RGS5) is increased in vascular smooth muscle cells (SMCs) of remodelling collateral arterioles. RGS5 terminates G-protein-coupled signalling cascades which control contractile responses of SMCs. Consequently, overexpression of RGS5 blunted Gαq/11-mediated mobilization of intracellular calcium, thereby facilitating Gα12/13-mediated RhoA signalling which is crucial for arteriogenesis. Knockdown of RGS5 evoked opposite effects and thus strongly impaired collateral growth as evidenced by a blockade of RhoA activation, SMC proliferation and the inability of these cells to acquire an activated phenotype in RGS5-deficient mice after the onset of arteriogenesis. Collectively, these findings establish RGS5 as a novel determinant of arteriogenesis which shifts G-protein signalling from Gαq/11-mediated calcium-dependent contraction towards Gα12/13-mediated Rho kinase-dependent SMC activation.
Keywords: G‐protein; RGS5; arteriogenesis; remodelling; vascular smooth muscle cells.
© 2014 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
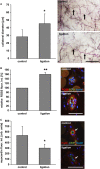
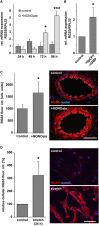
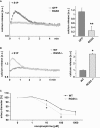
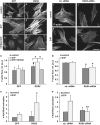


References
-
- Adams LD, Geary RL, McManus B, Schwartz SM. A comparison of aorta and vena cava medial message expression by cDNA array analysis identifies a set of 68 consistently differentially expressed genes, all in aortic media. Circ Res. 2000;87:623–631. - PubMed
-
- Banai S, Wolf Y, Golomb G, Pearle A, Waltenberger J, Fishbein I, Schneider A, Gazit A, Perez L, Huber R, et al. PDGF-receptor tyrosine kinase blocker AG1295 selectively attenuates smooth muscle cell growth in vitro and reduces neointimal formation after balloon angioplasty in swine. Circulation. 1998;97:1960–1969. - PubMed
-
- Berger M, Bergers G, Arnold B, Hammerling GJ, Ganss R. Regulator of G-protein signaling-5 induction in pericytes coincides with active vessel remodeling during neovascularization. Blood. 2005;105:1094–1101. - PubMed
-
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein alpha subunits. Cell. 1996;86:445–452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

