LitR is a repressor of syp genes and has a temperature-sensitive regulatory effect on biofilm formation and colony morphology in Vibrio (Aliivibrio) salmonicida
- PMID: 24973072
- PMCID: PMC4136084
- DOI: 10.1128/AEM.01239-14
LitR is a repressor of syp genes and has a temperature-sensitive regulatory effect on biofilm formation and colony morphology in Vibrio (Aliivibrio) salmonicida
Abstract
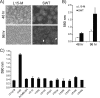
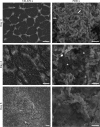
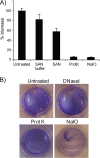
Vibrio (Aliivibrio) salmonicida is the etiological agent of cold water vibriosis, a disease in farmed Atlantic salmon (Salmo salar) that is kept under control due to an effective vaccine. A seawater temperature below 12°C is normally required for disease development. Quorum sensing (QS) is a cell density-regulated communication system that bacteria use to coordinate activities involved in colonization and pathogenesis, and we have previously shown that inactivation of the QS master regulator LitR attenuates the V. salmonicida strain LFI1238 in a fish model. We show here that strain LFI1238 and a panel of naturally occurring V. salmonicida strains are poor biofilm producers. Inactivation of litR in the LFI1238 strain enhances medium- and temperature-dependent adhesion, rugose colony morphology, and biofilm formation. Chemical treatment and electron microscopy of the biofilm identified an extracellular matrix consisting mainly of a fibrous network, proteins, and polysaccharides. Further, by microarray analysis of planktonic and biofilm cells, we identified a number of genes regulated by LitR and, among these, were homologues of the Vibrio fischeri symbiosis polysaccharide (syp) genes. The syp genes were regulated by LitR in both planktonic and biofilm lifestyle analyses. Disruption of syp genes in the V. salmonicida ΔlitR mutant alleviated adhesion, rugose colony morphology, and biofilm formation. Hence, LitR is a repressor of syp transcription that is necessary for expression of the phenotypes examined. The regulatory effect of LitR on colony morphology and biofilm formation is temperature sensitive and weak or absent at temperatures above the bacterium's upper threshold for pathogenicity.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

