Loss of α(E)-catenin potentiates cisplatin-induced nephrotoxicity via increasing apoptosis in renal tubular epithelial cells
- PMID: 24973089
- PMCID: PMC4271126
- DOI: 10.1093/toxsci/kfu130
Loss of α(E)-catenin potentiates cisplatin-induced nephrotoxicity via increasing apoptosis in renal tubular epithelial cells
Abstract
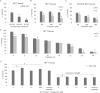
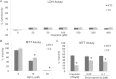
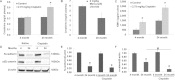
Cisplatin is one of the most potent and widely used antitumor drugs. However, the use of cisplatin is limited by its side effect, nephrotoxicity. Evidence has shown an increased incidence and severity of acute kidney injury (AKI) in the elderly. Previous studies from our laboratory demonstrate a decrease in α(E)-catenin expression in aged kidney. In this study, we investigated whether the loss of α(E)-catenin may increase cisplatin nephrotoxicity. To study the effects of reduced α(E)-catenin, a cell line with stable knockdown of α(E)-catenin (C2 cells) was used; NT3 is nontargeted control. C2 cells exhibited a significant loss of viability as determined by MTT assay compared with NT3 cells after cisplatin challenge, but showed no difference in lactate dehydrogenase (LDH) leakage. Increased caspase 3/7 activation and PARP cleavage was observed in C2 cells after cisplatin treatment. Z-VAD, a pan-caspase inhibitor, abolished the difference in susceptibility between NT3 and C2 cells. Interestingly, the expression of α(E)-catenin was further decreased after cisplatin treatment. Furthermore, in vivo data demonstrated a significant increase in serum creatinine at 72 h after a single dose of cisplatin in 24-month-old rats, but not in 4-month-old rats. Increased expression of KIM-1 and in situ apoptosis were also detected in aged kidney after cisplatin challenge. Taken together, these data suggest that loss of α(E)-catenin increases apoptosis of tubular epithelial cells which may contribute to the increased nephrotoxicity induced by cisplatin in aged kidney.
Keywords: AKI; aging; apoptosis; cisplatin; α-catenin.
© The Author 2014. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells.Apoptosis. 2015 Jul;20(7):921-9. doi: 10.1007/s10495-015-1129-x. Apoptosis. 2015. PMID: 25894537 Free PMC article.
-
Wnt/β-catenin agonist BIO alleviates cisplatin-induced nephrotoxicity without compromising its efficacy of anti-proliferation in ovarian cancer.Life Sci. 2020 Dec 15;263:118672. doi: 10.1016/j.lfs.2020.118672. Epub 2020 Oct 26. Life Sci. 2020. PMID: 33121990
-
3-deazaneplanocin A protects against cisplatin-induced renal tubular cell apoptosis and acute kidney injury by restoration of E-cadherin expression.Cell Death Dis. 2019 May 1;10(5):355. doi: 10.1038/s41419-019-1589-y. Cell Death Dis. 2019. PMID: 31043583 Free PMC article.
-
Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity.Autophagy. 2008 Jul;4(5):710-2. doi: 10.4161/auto.6309. Epub 2008 May 20. Autophagy. 2008. PMID: 18497570 Review.
-
Mechanisms of Cisplatin nephrotoxicity.Toxins (Basel). 2010 Nov;2(11):2490-518. doi: 10.3390/toxins2112490. Epub 2010 Oct 26. Toxins (Basel). 2010. PMID: 22069563 Free PMC article. Review.
Cited by
-
The renoprotective effects of soy protein in the aging rat kidney.Med Res Arch. 2020 Mar;8(3):10.18103/mra.v8i3.2065. doi: 10.18103/mra.v8i3.2065. Epub 2020 Mar 31. Med Res Arch. 2020. PMID: 34222651 Free PMC article.
-
The impact of aging on epithelial barriers.Tissue Barriers. 2017 Oct 2;5(4):e1343172. doi: 10.1080/21688370.2017.1343172. Epub 2017 Jul 7. Tissue Barriers. 2017. PMID: 28686506 Free PMC article. Review.
-
Twist2 Is Upregulated in Early Stages of Repair Following Acute Kidney Injury.Int J Mol Sci. 2017 Feb 10;18(2):368. doi: 10.3390/ijms18020368. Int J Mol Sci. 2017. PMID: 28208580 Free PMC article.
-
The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity.Int J Mol Sci. 2019 Oct 22;20(20):5238. doi: 10.3390/ijms20205238. Int J Mol Sci. 2019. PMID: 31652595 Free PMC article. Review.
-
Mechanism of action of novel piperazine containing a toxicant against human liver cancer cells.PeerJ. 2016 Mar 17;4:e1588. doi: 10.7717/peerj.1588. eCollection 2016. PeerJ. 2016. Retraction in: PeerJ. 2016 Jun 26;4:e1588/retraction. doi: 10.7717/peerj.1588/retraction. PMID: 27019772 Free PMC article. Retracted.
References
-
- Bellomo R., Kellum J. A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. - PubMed
-
- Desai R., Sarpal R., Ishiyama N., Pellikka M., Ikura M., Tepass U. Monomeric alpha-catenin links cadherin to the actin cytoskeleton. Nat. Cell Biol. 2013;15:261–273. - PubMed
-
- Devemy E., Blaschuk O. W. Identification of a novel dual E- and N-cadherin antagonist. Peptides. 2009;30:1539–1547. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

