Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis
- PMID: 24973208
- PMCID: PMC4132787
- DOI: 10.1074/jbc.M114.563064
Platelet-derived growth factor primes cancer-associated fibroblasts for apoptosis
Abstract
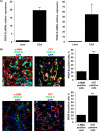
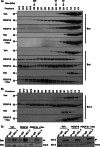
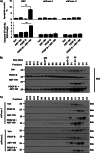
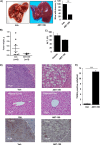
Desmoplastic malignancies such as cholangiocarcinoma (CCA) are characterized by a dense stroma containing an abundance of myofibroblasts termed cancer-associated fibroblasts (CAF). The CAF phenotype represents an "activated state" in which cells are primed for cell death triggered by BH3 mimetics. Accordingly, this primed state may be therapeutically exploited. To elucidate the mechanisms underlying this poorly understood apoptotic priming, we examined the role of platelet-derived growth factor (PDGF) in CAF priming for cell death given its prominent role in CAF activation. PDGF isomers PDGF-B and PDGF-D are abundantly expressed in CCA cells derived from human specimens. Either isomer sensitizes myofibroblasts to cell death triggered by BH3 mimetics. Similar apoptotic sensitization was observed with co-culture of myofibroblasts and CCA cells. Profiling of Bcl-2 proteins expressed by PDGF-primed myofibroblasts demonstrated an increase in cellular levels of Puma. PDGF-mediated increases in cellular Puma levels induced proapoptotic changes in Bak, which resulted in its binding to Bcl-2. Short hairpin RNA-mediated down-regulation of Puma conferred resistance to PDGF-mediated apoptotic priming. Conversely, the BH3 mimetic navitoclax disrupted Bcl-2/Bak heterodimers, allowing Bak to execute the cell death program. Treatment with a Bcl-2-specific BH3 mimetic, ABT-199, reduced tumor formation and tumor burden in a murine model of cholangiocarcinoma. Collectively, these findings indicate that apoptotic priming of CAF by PDGF occurs via Puma-mediated Bak activation, which can be converted to active full-blown apoptosis by navitoclax or ABT-199 for therapeutic benefit.
Keywords: Apoptosis; B-cell Lymphoma 2 (Bcl-2); BH3 Mimetics; Cancer Biology; Cholangiocarcinoma; Myofibroblast; PDGF; Tumor Microenvironment.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

