The serotonin transporter undergoes constitutive internalization and is primarily sorted to late endosomes and lysosomal degradation
- PMID: 24973209
- PMCID: PMC4132800
- DOI: 10.1074/jbc.M113.495754
The serotonin transporter undergoes constitutive internalization and is primarily sorted to late endosomes and lysosomal degradation
Abstract
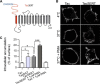
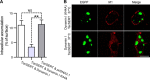
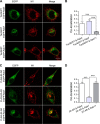
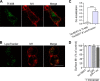
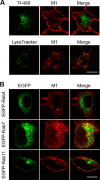
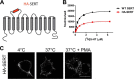
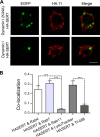
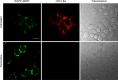
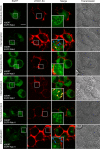
The serotonin transporter (SERT) plays a critical role in regulating serotonin signaling by mediating reuptake of serotonin from the extracellular space. The molecular and cellular mechanisms controlling SERT levels in the membrane remain poorly understood. To study trafficking of the surface resident SERT, two functional epitope-tagged variants were generated. Fusion of a FLAG-tagged one-transmembrane segment protein Tac to the SERT N terminus generated a transporter with an extracellular epitope suited for trafficking studies (TacSERT). Likewise, a construct with an extracellular antibody epitope was generated by introducing an HA (hemagglutinin) tag in the extracellular loop 2 of SERT (HA-SERT). By using TacSERT and HA-SERT in antibody-based internalization assays, we show that SERT undergoes constitutive internalization in a dynamin-dependent manner. Confocal images of constitutively internalized SERT demonstrated that SERT primarily co-localized with the late endosomal/lysosomal marker Rab7, whereas little co-localization was observed with the Rab11, a marker of the "long loop" recycling pathway. This sorting pattern was distinct from that of a prototypical recycling membrane protein, the β2-adrenergic receptor. Furthermore, internalized SERT co-localized with the lysosomal marker LysoTracker and not with transferrin. The sorting pattern was further confirmed by visualizing internalization of SERT using the fluorescent cocaine analog JHC1-64 and by reversible and pulse-chase biotinylation assays showing evidence for lysosomal degradation of the internalized transporter. Finally, we found that SERT internalized in response to stimulation with 12-myristate 13-acetate co-localized primarily with Rab7- and LysoTracker-positive compartments. We conclude that SERT is constitutively internalized and that the internalized transporter is sorted mainly to degradation.
Keywords: Cellular Regulation; Endocytosis; Intracellular Trafficking; Monoamine Transporter; Protein Kinase C (PKC); Protein Sorting; Serotonin Transporter.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Chen N. H., Reith M. E., Quick M. W. (2004) Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 447, 519–531 - PubMed
-
- Kristensen A. S., Andersen J., Jørgensen T. N., Sørensen L., Eriksen J., Loland C. J., Strømgaard K., Gether U. (2011) SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640 - PubMed
-
- Yamashita A., Singh S. K., Kawate T., Jin Y., Gouaux E. (2005) Crystal structure of a bacterial homologue of Na+/Cl−-dependent neurotransmitter transporters. Nature 437, 215–223 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

