Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability
- PMID: 24973213
- PMCID: PMC4139223
- DOI: 10.1074/jbc.M114.566927
Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability
Abstract
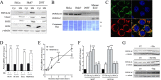
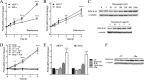
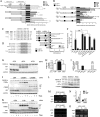
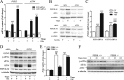
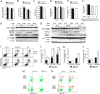
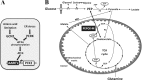
Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M), encoded by the nuclear PCK2 gene, links TCA cycle intermediates and glycolytic pools through the conversion of mitochondrial oxaloacetate into phosphoenolpyruvate. In the liver PEPCK-M adjoins its profusely studied cytosolic isoform (PEPCK-C) potentiating gluconeogenesis and TCA flux. However, PEPCK-M is present in a variety of non-gluconeogenic tissues, including tumors of several origins. Despite its potential relevance to cancer metabolism, the mechanisms responsible for PCK2 gene regulation have not been elucidated. The present study demonstrates PEPCK-M overexpression in tumorigenic cells as well as the mechanism for the modulation of PCK2 abundance under several stress conditions. Amino acid limitation and ER stress inducers, conditions that activate the amino acid response (AAR) and the unfolded protein response (UPR), stimulate PCK2 gene transcription. Both the AAR and UPR lead to increased synthesis of ATF4, which mediates PCK2 transcriptional up-regulation through its binding to a putative ATF/CRE composite site within the PCK2 promoter functioning as an amino acid response element. In addition, activation of the GCN2-eIF2α-ATF4 and PERK-eIF2α-ATF4 signaling pathways are responsible for increased PEPCK-M levels. Finally, PEPCK-M knockdown using either siRNA or shRNA were sufficient to reduce MCF7 mammary carcinoma cell growth and increase cell death under glutamine deprivation or ER stress conditions. Our data demonstrate that this enzyme has a critical role in the survival program initiated upon stress and shed light on an unexpected and important role of mitochondrial PEPCK in cancer metabolism.
Keywords: AAR; AARE; ATF4; Cell Metabolism; ER Stress; PEPCK-M; Transcription Regulation; Tumor Metabolism; UPR; Unfolded Protein Response (UPR).
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Chang H. C., Lane M. D. (1966) The enzymatic carboxylation of phosphoenolpyruvate. II. Purification and properties of liver mitochondrial phosphoenolpyruvate carboxykinase. J. Biol. Chem. 241, 2413–2420 - PubMed
-
- Nordlie R. C., Lardy H. A. (1963) Mammalian liver phosphoneolpyruvate carboxykinase activities. J. Biol. Chem. 238, 2259–2263 - PubMed
-
- Hanson R. W., Garber A. J. (1972) Phosphoenolpyruvate carboxykinase: I. Its role in gluconeogenesis. Am. J. Clin. Nutr. 25, 1010–1021 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

