Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme
- PMID: 24973216
- PMCID: PMC4148879
- DOI: 10.1074/jbc.M114.584037
Structural basis of transcription initiation by bacterial RNA polymerase holoenzyme
Abstract
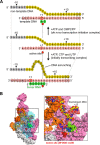
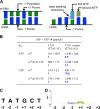
The bacterial RNA polymerase (RNAP) holoenzyme containing σ factor initiates transcription at specific promoter sites by de novo RNA priming, the first step of RNA synthesis where RNAP accepts two initiating ribonucleoside triphosphates (iNTPs) and performs the first phosphodiester bond formation. We present the structure of de novo transcription initiation complex that reveals unique contacts of the iNTPs bound at the transcription start site with the template DNA and also with RNAP and demonstrate the importance of these contacts for transcription initiation. To get further insight into the mechanism of RNA priming, we determined the structure of initially transcribing complex of RNAP holoenzyme with 6-mer RNA, obtained by in crystallo transcription approach. The structure highlights RNAP-RNA contacts that stabilize the short RNA transcript in the active site and demonstrates that the RNA 5'-end displaces σ region 3.2 from its position near the active site, which likely plays a key role in σ ejection during the initiation-to-elongation transition. Given the structural conservation of the RNAP active site, the mechanism of de novo RNA priming appears to be conserved in all cellular RNAPs.
Keywords: Promoter; RNA Polymerase; Transcription; Transcription Initiation Factor; X-ray Crystallography.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Vassylyev D. G., Vassylyeva M. N., Zhang J., Palangat M., Artsimovitch I., Landick R. (2007) Structural basis for substrate loading in bacterial RNA polymerase. Nature 448, 163–168 - PubMed
-
- McClure W. R., Cech C. L., Johnston D. E. (1978) A steady state assay for the RNA polymerase initiation reaction. J. Biol. Chem. 253, 8941–8948 - PubMed
-
- Kulbachinskiy A., Mustaev A. (2006) Region 3.2 of the sigma subunit contributes to the binding of the 3′-initiating nucleotide in the RNA polymerase active center and facilitates promoter clearance during initiation. J. Biol. Chem. 281, 18273–18276 - PubMed
-
- Gaal T., Bartlett M. S., Ross W., Turnbough C. L., Jr., Gourse R. L. (1997) Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science 278, 2092–2097 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

