β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent
- PMID: 24973219
- PMCID: PMC4132805
- DOI: 10.1074/jbc.M114.557652
β-Agonist-mediated relaxation of airway smooth muscle is protein kinase A-dependent
Abstract
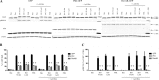
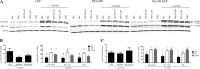
Inhaled β-agonists are effective at reversing bronchoconstriction in asthma, but the mechanism by which they exert this effect is unclear and controversial. PKA is the historically accepted effector, although this assumption is made on the basis of associative and not direct evidence. Recent studies have asserted that exchange protein activated by cAMP (Epac), not PKA, mediates the relaxation of airway smooth muscle (ASM) observed with β-agonist treatment. This study aims to clarify the role of PKA in the prorelaxant effects of β-agonists on ASM. Inhibition of PKA activity via expression of the PKI and RevAB peptides results in increased β-agonist-mediated cAMP release, abolishes the inhibitory effect of isoproterenol on histamine-induced intracellular calcium flux, and significantly attenuates histamine-stimulated MLC-20 phosphorylation. Analyses of ASM cell and tissue contraction demonstrate that PKA inhibition eliminates most, if not all, β-agonist-mediated relaxation of contracted smooth muscle. Conversely, Epac knockdown had no effect on the regulation of contraction or procontractile signaling by isoproterenol. These findings suggest that PKA, not Epac, is the predominant and physiologically relevant effector through which β-agonists exert their relaxant effects.
Keywords: Adrenergic Receptor; Asthma; G Protein-coupled Receptor (GPCR); Protein Kinase A (PKA); Smooth Muscle.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures






References
-
- Newnham D. M., McDevitt D. G., Lipworth B. J. (1994) Bronchodilator subsensitivity after chronic dosing with eformoterol in patients with asthma. The American Journal of Medicine 97, 29–37 - PubMed
-
- Grove A., Lipworth B. J. (1995) Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet 346, 201–206 - PubMed
-
- Sears M. R. (2002) Adverse effects of b-agonists. J. Allergy Clin. Immunol. 110, S322–S328 - PubMed
-
- Salpeter S. R., Buckley N. S., Ormiston T. M., Salpeter E. E. (2006) Meta-analysis: effect of long-acting b-Agonists on severe asthma exacerbations and asthma-related deaths. Annals Intern. Med. 144, 904–912 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058506/HL/NHLBI NIH HHS/United States
- R00 HL087560/HL/NHLBI NIH HHS/United States
- HL114471/HL/NHLBI NIH HHS/United States
- P01 HL066299/HL/NHLBI NIH HHS/United States
- R29 HL058506/HL/NHLBI NIH HHS/United States
- P30 ES013508/ES/NIEHS NIH HHS/United States
- HL58506/HL/NHLBI NIH HHS/United States
- R01 AG041265/AG/NIA NIH HHS/United States
- HL087560/HL/NHLBI NIH HHS/United States
- P30 CA056036/CA/NCI NIH HHS/United States
- P01 HL114471/HL/NHLBI NIH HHS/United States
- T32 AR07592/AR/NIAMS NIH HHS/United States
- K99 HL087560/HL/NHLBI NIH HHS/United States
- AG041265/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

