Galatrox is a C-type lectin in Bothrops atrox snake venom that selectively binds LacNAc-terminated glycans and can induce acute inflammation
- PMID: 24973254
- PMCID: PMC4181450
- DOI: 10.1093/glycob/cwu061
Galatrox is a C-type lectin in Bothrops atrox snake venom that selectively binds LacNAc-terminated glycans and can induce acute inflammation
Abstract
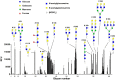
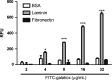
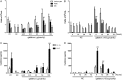
Previous studies indicate that snake venom contains glycan-binding proteins (GBPs), although the binding specificity and biological activities of many of these GBPs is unclear. Here we report our studies on the glycan binding specificity and activities of galatrox, a Bothrops atrox snake venom-derived GBP. Glycan microarray analysis indicates that galatrox binds most strongly to glycans expressing N-acetyllactosamine (LacNAc), with a significant preference for Galβ1-4GlcNAcβ over Galβ1-3GlcNAcβ compounds. Galatrox also bound immobilized laminin, a LacNAc-dense extracellular matrix component, suggesting that this GBP can bind LacNAc-bearing glycoproteins. As several endogenous mammalian GBPs utilize a similar binding LacNAc binding preference to regulate neutrophil and monocyte activity, we hypothesized that galatrox may mediate B. atrox toxicity through regulation of leukocyte activity. Indeed, galatrox bound neutrophils and promoted leukocyte chemotaxis in a carbohydrate-dependent manner. Similarly, galatrox administration into the mouse peritoneal cavity induced significant neutrophil migration and the release of pro-inflammatory cytokines IL-1α and IL-6. Exposure of bone marrow-derived macrophages to galatrox induced generation of pro-inflammatory mediators IL-6, TNF-α, and keratinocyte-derived chemokine. This signaling by galatrox was mediated via its carbohydrate recognition domain by activation of the TLR4-mediated MyD88-dependent signaling pathway. These results indicate that galatrox has pro-inflammatory activity through its interaction with LacNAc-bearing glycans on neutrophils, macrophages and extracellular matrix proteins and induce the release of pro-inflammatory mediators.
Keywords: Bothrops atrox; C-type lectin; Inflammatory response; Neutrophil migration; Snake venom.
© The Author 2014. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


Similar articles
-
Isolation, functional, and partial biochemical characterization of galatrox, an acidic lectin from Bothrops atrox snake venom.Acta Biochim Biophys Sin (Shanghai). 2011 Mar;43(3):181-92. doi: 10.1093/abbs/gmr003. Epub 2011 Feb 4. Acta Biochim Biophys Sin (Shanghai). 2011. PMID: 21297119
-
Snake venom galactoside-binding lectin from Bothrops jararacussu: Special role in leukocytes activation and function.Int J Biol Macromol. 2025 Mar;296:139742. doi: 10.1016/j.ijbiomac.2025.139742. Epub 2025 Jan 10. Int J Biol Macromol. 2025. PMID: 39798729 Review.
-
The crucial role of the MyD88 adaptor protein in the inflammatory response induced by Bothrops atrox venom.Toxicon. 2013 Jun 1;67:37-46. doi: 10.1016/j.toxicon.2013.02.010. Epub 2013 Mar 5. Toxicon. 2013. PMID: 23474268
-
Release of Cytokines in the Peritoneal Fluid of C57BL/6 Mice After Bothrops jararaca and Bothrops atrox Venom Injection.Toxins (Basel). 2025 Mar 26;17(4):164. doi: 10.3390/toxins17040164. Toxins (Basel). 2025. PMID: 40278662 Free PMC article.
-
Inflammation induced by Bothrops asper venom.Toxicon. 2009 Jul;54(1):67-76. doi: 10.1016/j.toxicon.2009.03.019. Epub 2009 Mar 27. Toxicon. 2009. PMID: 19328821 Review.
Cited by
-
Correlating Fibrinogen Consumption and Profiles of Inflammatory Molecules in Human Envenomation's by Bothrops atrox in the Brazilian Amazon.Front Immunol. 2020 Aug 18;11:1874. doi: 10.3389/fimmu.2020.01874. eCollection 2020. Front Immunol. 2020. PMID: 32973773 Free PMC article.
-
An Immunological Stairway to Severe Tissue Complication Assembly in Bothrops atrox Snakebites.Front Immunol. 2019 Aug 13;10:1882. doi: 10.3389/fimmu.2019.01882. eCollection 2019. Front Immunol. 2019. PMID: 31474982 Free PMC article.
-
Glycobiology in osteoclast differentiation and function.Bone Res. 2023 Oct 26;11(1):55. doi: 10.1038/s41413-023-00293-6. Bone Res. 2023. PMID: 37884496 Free PMC article. Review.
-
Insights into the Role of Proteolytic and Adhesive Domains of Snake Venom Metalloproteinases from Bothrops spp. in the Control of Toxoplasma gondii Infection.Toxins (Basel). 2025 Feb 18;17(2):95. doi: 10.3390/toxins17020095. Toxins (Basel). 2025. PMID: 39998112 Free PMC article.
-
Snake venom galactoside-binding lectins: a structural and functional overview.J Venom Anim Toxins Incl Trop Dis. 2015 Sep 24;21:35. doi: 10.1186/s40409-015-0038-3. eCollection 2015. J Venom Anim Toxins Incl Trop Dis. 2015. PMID: 26413085 Free PMC article.
References
-
- Allavena P, Chieppa M, Monti P, Piemonti L. From pattern recognition receptor to regulator of homeostasis: The double-faced macrophage mannose receptor. Crit Rev Immunol. 2004;24:179–192. - PubMed
-
- Almkvist J, Dahlgren C, Leffler H, Karlsson A. Activation of the neutrophil nicotinamide adenine dinucleotide phosphate oxidase by galectin-1. J Immunol. 2002;168:4034–4041. - PubMed
-
- Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, Beyaert R, Pshezhetsky AV, Szewczuk MR. Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22:314–324. - PubMed
-
- Apostolakis S, Lip GY, Shantsila E. Monocytes in heart failure: Relationship to a deteriorating immune overreaction or a desperate attempt for tissue repair? Cardiovasc Res. 2010;85:649–660. - PubMed
-
- Ariel A, Timor O. Hanging in the balance: Endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol. 2013;229:250–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

