Building a flagellum outside the bacterial cell
- PMID: 24973293
- PMCID: PMC4183434
- DOI: 10.1016/j.tim.2014.05.009
Building a flagellum outside the bacterial cell
Abstract
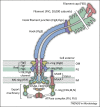
Flagella, the helical propellers that extend from the bacterial surface, are a paradigm for how complex molecular machines can be built outside the living cell. Their assembly requires ordered export of thousands of structural subunits across the cell membrane and this is achieved by a type III export machinery located at the flagellum base, after which subunits transit through a narrow channel at the core of the flagellum to reach the assembly site at the tip of the nascent structure, up to 20μm from the cell surface. Here we review recent findings that provide new insights into flagellar export and assembly, and a new and unanticipated mechanism for constant rate flagellum growth.
Keywords: bacterial flagellum; cell motility; chain mechanism; protein export; rotary nanomotor; type III secretion system.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Snyder L.A.S. Bacterial flagellar diversity and evolution: seek simplicity and distrust it? Trends Microbiol. 2009;17:1–5. - PubMed
-
- Berg H.C. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 2003;72:19–54. - PubMed
-
- Roberts M.A.J. Adaptation and control circuits in bacterial chemotaxis. Biochem. Soc. Trans. 2010;38:1265–1269. - PubMed
-
- Sowa Y., Berry R.M. The bacterial flagellar motor. Single Mol. Biol. 2009:105–142.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

