Vasohibin-1 deficiency enhances renal fibrosis and inflammation after unilateral ureteral obstruction
- PMID: 24973329
- PMCID: PMC4208642
- DOI: 10.14814/phy2.12054
Vasohibin-1 deficiency enhances renal fibrosis and inflammation after unilateral ureteral obstruction
Abstract
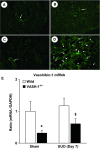
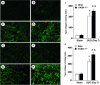
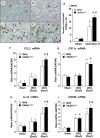
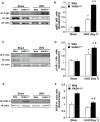
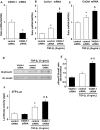
Tubulointerstitial injuries are known to predict the deterioration of renal function in chronic kidney disease (CKD). We recently reported the protective role of Vasohibin-1(VASH-1), a negative feedback regulator of angiogenesis, in diabetic nephropathy, but its impact on tubulointerstitial injuries remains to be elucidated. In the present study, we evaluated the role of endogenous VASH-1 in regulating the tubulointerstitial alterations induced by unilateral ureteral obstruction (UUO), and assessed its role on fibrogenesis and the activation of Smad3 signaling in renal fibroblasts. UUO was induced in female Vasohibin-1 heterozygous knockout mice (VASH-1(+/-)) or wild-type (WT) (VASH-1(+/+)) littermates. Mice were sacrificed on Day 7 after left ureter ligation, and the kidney tissue was obtained. Interstitial fibrosis, the accumulation of type I and type III collagen and monocytes/macrophages infiltration in the obstructed kidneys (OBK) were significantly exacerbated in VASH-1(+/-) mice compared with WT mice (Day 7). The increases in the renal levels of TGF-β1, pSmad3, NF-κB pp65, CCL2 mRNA, and the number of interstitial fibroblast-specific protein-1 (FSP-1)(+) fibroblasts in the OBK were significantly aggravated in VASH-1(+/-) mice. In addition, treatment with VASH-1 siRNA enhanced the TGF-β1-induced phosphorylation of Smad3, the transcriptional activation of the Smad3 pathway and the production of type I/type III collagen in fibroblasts, in vitro. Taken together, our findings demonstrate a protective role for endogenous VASH-1 on tubulointerstitial alterations via its regulation of inflammation and fibrosis and also show the direct anti-fibrotic effects of VASH-1 on renal fibroblasts through its modulation of TGF-β1 signaling.
Keywords: Inflammation; TGF‐β1; macrophage; tubulointerstitial fibrosis; unilateral ureteral obstruction; vasohibin‐1.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures




Similar articles
-
MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway.Int J Mol Sci. 2024 May 11;25(10):5232. doi: 10.3390/ijms25105232. Int J Mol Sci. 2024. PMID: 38791272 Free PMC article.
-
Sustained-release prostacyclin analog ONO-1301 ameliorates tubulointerstitial alterations in a mouse obstructive nephropathy model.Am J Physiol Renal Physiol. 2012 Jun 15;302(12):F1616-29. doi: 10.1152/ajprenal.00538.2011. Epub 2012 Mar 14. Am J Physiol Renal Physiol. 2012. PMID: 22419696
-
Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction.J Clin Invest. 2003 Nov;112(10):1486-94. doi: 10.1172/JCI19270. J Clin Invest. 2003. PMID: 14617750 Free PMC article.
-
Anti-renal fibrosis effect of asperulosidic acid via TGF-β1/smad2/smad3 and NF-κB signaling pathways in a rat model of unilateral ureteral obstruction.Phytomedicine. 2019 Feb;53:274-285. doi: 10.1016/j.phymed.2018.09.009. Epub 2018 Sep 5. Phytomedicine. 2019. PMID: 30668407
-
The Genomic Response to TGF-β1 Dictates Failed Repair and Progression of Fibrotic Disease in the Obstructed Kidney.Front Cell Dev Biol. 2021 Jul 2;9:678524. doi: 10.3389/fcell.2021.678524. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34277620 Free PMC article. Review.
Cited by
-
Diabetic kidney disease in type 2 diabetes: a review of pathogenic mechanisms, patient-related factors and therapeutic options.PeerJ. 2021 Apr 19;9:e11070. doi: 10.7717/peerj.11070. eCollection 2021. PeerJ. 2021. PMID: 33976959 Free PMC article.
-
Deletion of pro-angiogenic factor vasohibin-2 ameliorates glomerular alterations in a mouse diabetic nephropathy model.PLoS One. 2018 Apr 11;13(4):e0195779. doi: 10.1371/journal.pone.0195779. eCollection 2018. PLoS One. 2018. PMID: 29641565 Free PMC article.
-
Bone marrow‑derived mesenchymal stem cell‑conditioned medium attenuates tubulointerstitial fibrosis by inhibiting monocyte mobilization in an irreversible model of unilateral ureteral obstruction.Mol Med Rep. 2018 Jun;17(6):7701-7707. doi: 10.3892/mmr.2018.8848. Epub 2018 Apr 5. Mol Med Rep. 2018. PMID: 29620281 Free PMC article.
-
Mechanism of ELL-associated factor 2 and vasohibin 1 regulating invasion, migration, and angiogenesis in colorectal cancer.World J Gastroenterol. 2023 Jun 28;29(24):3770-3792. doi: 10.3748/wjg.v29.i24.3770. World J Gastroenterol. 2023. PMID: 37426316 Free PMC article.
-
MicroRNA-10 Family Promotes Renal Fibrosis through the VASH-1/Smad3 Pathway.Int J Mol Sci. 2024 May 11;25(10):5232. doi: 10.3390/ijms25105232. Int J Mol Sci. 2024. PMID: 38791272 Free PMC article.
References
-
- Bottinger E. P., Bitzer M. 2002. TGF‐beta signaling in renal disease. J. Am. Soc. Nephrol.; 13:2600-2610. - PubMed
-
- Chen S. J., Yuan W., Mori Y., Levenson A., Trojanowska M., Varga J. 1999. Stimulation of type I collagen transcription in human skin fibroblasts by TGF‐beta: involvement of Smad 3. J. Invest. Dermatol.; 112:49-57. - PubMed
-
- Chung A. C., Huang X. R., Zhou L., Heuchel R., Lai K. N., Lan H. Y. 2009. Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol. Dial. Transplant.; 24:1443-1454. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

