SGLT2 inhibitors act from the extracellular surface of the cell membrane
- PMID: 24973332
- PMCID: PMC4208661
- DOI: 10.14814/phy2.12058
SGLT2 inhibitors act from the extracellular surface of the cell membrane
Abstract
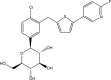
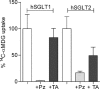
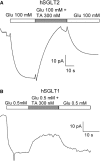
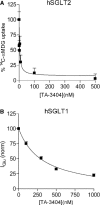
SGLT2 inhibitors are a new class of drugs that have been recently developed to treat type II diabetes. They lower glucose levels by inhibiting the renal Na(+)/glucose cotransporter SGLT2, thereby increasing the amount of glucose excreted in the urine. Pharmacodynamics studies have raised questions about how these inhibitors reach SGLT2 in the brush border membrane of the S1 and S2 segments of the renal proximal tubule: are these drugs filtered by the glomerulus and act extracellularly, or do they enter the cell and act intracellularly? To address this question we expressed hSGLT2 in HEK-293T cells and determined the affinity of a specific hSGLT2 inhibitor, TA-3404 (also known as JNJ-30980924), from the extra- and intracellular side of the plasma membrane. Inhibition of SGLT2 activity (Na(+)/glucose currents) by TA-3404 was determined using the whole-cell patch clamp that allows controlling the composition of both the extracellular and intracellular solutions. We compared the results to those obtained using the nonselective SGLT inhibitor phlorizin, and to the effect of TA-3404 on hSGLT1. Our results showed that TA-3404 is a potent extracellular inhibitor of glucose inward SGLT2 transport (IC50 2 nmol/L) but it was ineffective from the intracellular compartment at both low (5 mmol/L) and high (150 mmol/L) intracellular NaCl concentrations. We conclude that TA-3404 only inhibits SGLT2 from the extracellular side of the plasma membrane, suggesting that it is filtered from the blood through the glomerulus and acts from within the tubule lumen.
Keywords: Inhibitors; SGLT2; type II diabetes.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures
References
-
- Abdul‐Ghani M. A., Norton L., DeFronzo R. A. 2012. Efficacy and safety of SGLT2 inhibitors in the treatment of type 2 diabetes mellitus. Curr. Diab. Rep.; 2:230-238. - PubMed
-
- Devineni D., Curtin C. R., Polidori D., Gutierrez M. J., Murphy J., Rusch S. 2013. Pharmacokinetics and pharmacodynamics of canagliflozin, a sodium glucose co‐transporter 2 inhibitor, in subjects with type 2 diabetes mellitus. J. Clin. Pharmacol.; 53:601-610. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

