The effect of hypercapnia on static cerebral autoregulation
- PMID: 24973333
- PMCID: PMC4208638
- DOI: 10.14814/phy2.12059
The effect of hypercapnia on static cerebral autoregulation
Abstract
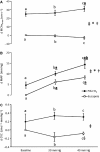
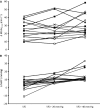
Hypercapnia impairs cerebrovascular control during rapid changes in blood pressure (BP); however, data concerning the effect of hypercapnia on steady state, nonpharmacological increases in BP is scarce. We recruited fifteen healthy volunteers (mean ± SD: age, 28 ± 6 years; body mass, 77 ± 12 kg) to assess the effect of hypercapnia on cerebrovascular control during steady-state elevations in mean arterial BP (MAP), induced via lower body positive pressure (LBPP). Following 20 min of supine rest, participants completed 5 min of eucapnic 20 and 40 mm Hg LBPP (order randomized) followed by 5 min of hypercapnia (5% CO2 in air) with and without LBPP (order randomized), and each stage was separated by ≥5 min to allow for recovery. Middle cerebral artery blood velocity (MCAv), BP, partial pressure of end-tidal carbon dioxide (PETCO2) and heart rate were recorded and presented as the change from the preceding baseline. No difference in MCAv was apparent between eupcapnic baseline and LBPPs (grouped mean 65 ± 11 cm·s(-1), all P > 0.05), despite the increased MAP with LBPP (Δ6 ± 5 and Δ8 ± 3 mm Hg for 20 and 40 mm Hg, respectively, both P < 0.001 vs. baseline). Conversely, MCAv during the hypercapnic +40 mm Hg stage (Δ31 ± 13 cm·s(-1)) was greater than hypercapnia alone (Δ25 ± 11 cm·s(-1), P = 0.026), due to an increased MAP (Δ14 ± 7 mm Hg, P < 0.001 vs. hypercapnia alone and P = 0.026 vs. hypercapnia +20 mm Hg). As cardiac output and PETCO2 were similar across all hypercapnic stages (all P > 0.05), our findings indicate that hypercapnia impairs static autoregulation, such that higher blood pressures are translated into the cerebral circulation.
Keywords: Cerebral blood flow; hypercapnia; lower body positive pressure; static cerebral autoregulation.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

References
-
- Aaslid R., Lindegaard K. F., Sorteberg W., Nornes H. 1989. Cerebral autoregulation dynamics in humans. Stroke; 20:45-52. - PubMed
-
- Ainslie P. N., Duffin J. 2009. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol.; 296:R1473-R1495. - PubMed
-
- Bill A., Linder J. 1976. Sympathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiol. Scand.; 96:114-121. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

