Activation and propagation of Ca2+ release from inside the sarcoplasmic reticulum network of mammalian skeletal muscle
- PMID: 24973406
- PMCID: PMC4192700
- DOI: 10.1113/jphysiol.2014.274274
Activation and propagation of Ca2+ release from inside the sarcoplasmic reticulum network of mammalian skeletal muscle
Abstract
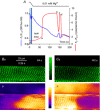
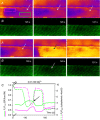
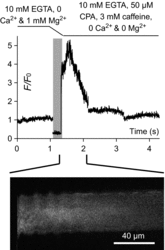
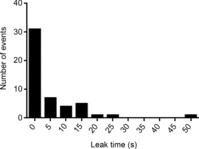
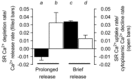
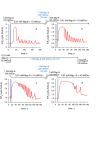
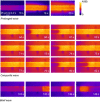
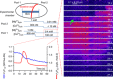
Skeletal muscle fibres are large and highly elongated cells specialized for producing the force required for posture and movement. The process of controlling the production of force within the muscle, known as excitation-contraction coupling, requires virtually simultaneous release of large amounts of Ca(2+) from the sarcoplasmic reticulum (SR) at the level of every sarcomere within the muscle fibre. Here we imaged Ca(2+) movements within the SR, tubular (t-) system and in the cytoplasm to observe that the SR of skeletal muscle is a connected network capable of allowing diffusion of Ca(2+) within its lumen to promote the propagation of Ca(2+) release throughout the fibre under conditions where inhibition of SR ryanodine receptors (RyRs) was reduced. Reduction of cytoplasmic [Mg(2+)] ([Mg(2+)]cyto) induced a leak of Ca(2+) through RyRs, causing a reduction in SR Ca(2+) buffering power argued to be due to a breakdown of SR calsequestrin polymers, leading to a local elevation of [Ca(2+)]SR. The local rise in [Ca(2+)]SR, an intra-SR Ca(2+) transient, induced a local diffusely rising [Ca(2+)]cyto. A prolonged Ca(2+) wave lasting tens of seconds or more was generated from these events. Ca(2+) waves were dependent on the diffusion of Ca(2+) within the lumen of the SR and ended as [Ca(2+)]SR dropped to low levels to inactivate RyRs. Inactivation of RyRs allowed re-accumulation of [Ca(2+)]SR and the activation of secondary Ca(2+) waves in the persistent presence of low [Mg(2+)]cyto if the threshold [Ca(2+)]SR for RyR opening could be reached. Secondary Ca(2+) waves occurred without an abrupt reduction in SR Ca(2+) buffering power. Ca(2+) release and wave propagation occurred in the absence of Ca(2+)-induced Ca(2+) release. These observations are consistent with the activation of Ca(2+) release through RyRs of lowered cytoplasmic inhibition by [Ca(2+)]SR or store overload-induced Ca(2+) release. Restitution of SR Ca(2+) buffering power to its initially high value required imposing normal resting ionic conditions in the cytoplasm, which re-imposed the normal resting inhibition on the RyRs, allowing [Ca(2+)]SR to return to endogenous levels without activation of store overload-induced Ca(2+) release. These results are discussed in the context of how pathophysiological Ca(2+) release such as that occurring in malignant hyperthermia can be generated.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures



Similar articles
-
Leaky ryanodine receptors delay the activation of store overload-induced Ca2+ release, a mechanism underlying malignant hyperthermia-like events in dystrophic muscle.Am J Physiol Cell Physiol. 2016 Apr 15;310(8):C673-80. doi: 10.1152/ajpcell.00366.2015. Epub 2016 Jan 28. Am J Physiol Cell Physiol. 2016. PMID: 26825125
-
A novel mechanism of tandem activation of ryanodine receptors by cytosolic and SR luminal Ca2+ during excitation-contraction coupling in atrial myocytes.J Physiol. 2017 Jun 15;595(12):3835-3845. doi: 10.1113/JP273611. Epub 2017 Feb 1. J Physiol. 2017. PMID: 28028837 Free PMC article.
-
Reorganized stores and impaired calcium handling in skeletal muscle of mice lacking calsequestrin-1.J Physiol. 2007 Sep 1;583(Pt 2):767-84. doi: 10.1113/jphysiol.2007.138024. Epub 2007 Jul 12. J Physiol. 2007. PMID: 17627988 Free PMC article.
-
Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites.Clin Exp Pharmacol Physiol. 2007 Sep;34(9):889-96. doi: 10.1111/j.1440-1681.2007.04708.x. Clin Exp Pharmacol Physiol. 2007. PMID: 17645636 Review.
-
Ryanodine receptor activity and store-operated Ca2+ entry: Critical regulators of Ca2+ content and function in skeletal muscle.J Physiol. 2023 Oct;601(19):4183-4202. doi: 10.1113/JP279512. Epub 2022 Mar 16. J Physiol. 2023. PMID: 35218018 Review.
Cited by
-
Superoxide Anions Inhibit Intracellular Calcium Response in Porcine Airway Smooth Muscle Cells.AJP Rep. 2024 May 23;14(2):e162-e169. doi: 10.1055/a-2318-0625. eCollection 2024 Apr. AJP Rep. 2024. PMID: 38784940 Free PMC article.
-
Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle.Proc Natl Acad Sci U S A. 2017 Jan 24;114(4):E638-E647. doi: 10.1073/pnas.1620265114. Epub 2017 Jan 9. Proc Natl Acad Sci U S A. 2017. PMID: 28069951 Free PMC article.
-
From synaptic input to muscle contraction: arm muscle cells of Octopus vulgaris show unique neuromuscular junction and excitation-contraction coupling properties.Proc Biol Sci. 2019 Aug 28;286(1909):20191278. doi: 10.1098/rspb.2019.1278. Epub 2019 Aug 28. Proc Biol Sci. 2019. PMID: 31455193 Free PMC article.
-
Evolutionary isolation of ryanodine receptor isoform 1 for muscle-based thermogenesis in mammals.Proc Natl Acad Sci U S A. 2023 Jan 24;120(4):e2117503120. doi: 10.1073/pnas.2117503120. Epub 2023 Jan 17. Proc Natl Acad Sci U S A. 2023. PMID: 36649401 Free PMC article.
-
Observation of the molecular organization of calcium release sites in fast- and slow-twitch skeletal muscle with nanoscale imaging.J R Soc Interface. 2014 Oct 6;11(99):20140570. doi: 10.1098/rsif.2014.0570. J R Soc Interface. 2014. PMID: 25100314 Free PMC article.
References
-
- Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. - PubMed
-
- Bandschapp O, Girard T. Malignant hyperthermia. Swiss Med Wkly. 2012;142:w13652. - PubMed
-
- Beard NA, Wei L, Dulhunty AF. Control of muscle ryanodine receptor calcium release channels by proteins in the sarcoplasmic reticulum lumen. Clin Exp Pharmacol Physiol. 2009;36:340–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

