Conventional but not plasmacytoid dendritic cells foster the systemic virus-induced type I IFN response needed for efficient CD8 T cell priming
- PMID: 24973449
- PMCID: PMC4105236
- DOI: 10.4049/jimmunol.1301440
Conventional but not plasmacytoid dendritic cells foster the systemic virus-induced type I IFN response needed for efficient CD8 T cell priming
Abstract
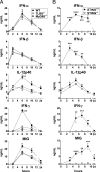
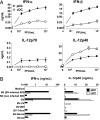
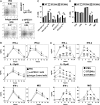
Plasmacytoid dendritic cells (pDCs) are considered to be the principal type-I IFN (IFN-I) source in response to viruses, whereas the contribution of conventional DCs (cDCs) has been underestimated because, on a per-cell basis, they are not considered professional IFN-I-producing cells. We have investigated their respective roles in the IFN-I response required for CTL activation. Using a nonreplicative virus, baculovirus, we show that despite the high IFN-I-producing abilities of pDCs, in vivo cDCs but not pDCs are the pivotal IFN-I producers upon viral injection, as demonstrated by selective pDC or cDC depletion. The pathway involved in the virus-triggered IFN-I response is dependent on TLR9/MyD88 in pDCs and on stimulator of IFN genes (STING) in cDCs. Importantly, STING is the key molecule for the systemic baculovirus-induced IFN-I response required for CTL priming. The supremacy of cDCs over pDCs in fostering the IFN-I response required for CTL activation was also verified in the lymphocytic choriomeningitis virus model, in which IFN-β promoter stimulator 1 plays the role of STING. However, when the TLR-independent virus-triggered IFN-I production is impaired, the pDC-induced IFNs-I have a primary impact on CTL activation, as shown by the detrimental effect of pDC depletion and IFN-I signaling blockade on the residual lymphocytic choriomeningitis virus-triggered CTL response detected in IFN-β promoter stimulator 1(-/-) mice. Our findings reveal that cDCs play a major role in the TLR-independent virus-triggered IFN-I production required for CTL priming, whereas pDC-induced IFNs-I are dispensable but become relevant when the TLR-independent IFN-I response is impaired.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures




References
-
- Reis e Sousa C. 2004. Activation of dendritic cells: translating innate into adaptive immunity. Curr. Opin. Immunol. 16: 21–25 - PubMed
-
- Lee H. K., Iwasaki A.. 2007. Innate control of adaptive immunity: dendritic cells and beyond. Semin. Immunol. 19: 48–55 - PubMed
-
- Mescher M. F., Curtsinger J. M., Agarwal P., Casey K. A., Gerner M., Hammerbeck C. D., Popescu F., Xiao Z.. 2006. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211: 81–92 - PubMed
-
- Santini S. M., Di Pucchio T., Lapenta C., Parlato S., Logozzi M., Belardelli F.. 2002. The natural alliance between type I interferon and dendritic cells and its role in linking innate and adaptive immunity. J. Interferon Cytokine Res. 22: 1071–1080 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

