The opioid receptor pharmacology of GSK1521498 compared to other ligands with differential effects on compulsive reward-related behaviours
- PMID: 24973897
- PMCID: PMC4281354
- DOI: 10.1007/s00213-014-3666-3
The opioid receptor pharmacology of GSK1521498 compared to other ligands with differential effects on compulsive reward-related behaviours
Abstract
Rationale: The novel opioid receptor antagonist, GSK1421498, has been shown to attenuate reward-driven compulsive behaviours, such as stimulant drug seeking or binge eating, in animals and humans. Here, we report new data on the receptor pharmacology of GSK121498, in comparison to naltrexone, naloxone, 6-β-naltrexol and nalmefene.
Objectives: To determine whether the novel opioid antagonist, GSK1521498, is an orthosteric or allosteric antagonist at the μ opioid receptor (MOPr) and whether it has neutral antagonist or inverse agonist properties.
Methods: A combination of radioligand binding assays and [(35)S]GTPγS binding assays was employed.
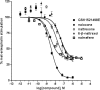
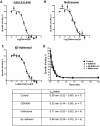
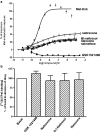
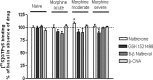
Results: GSK1521498 completely displaced [(3)H]naloxone binding to MOPr and did not alter the rate of [(3)H]naloxone dissociation from MOPr observations compatible with it binding to the orthosteric site on MOPr. GSK1521498 exhibited inverse agonism when MOPr was overexpressed but not when the level of MOPr expression was low. In parallel studies under conditions of high receptor expression density, naloxone, naltrexone, 6-β-naltrexol and nalmefene exhibited partial agonism, not inverse agonism as has been reported previously for naloxone and naltrexone. In brain tissue from mice receiving a prolonged morphine pre-treatment, GSK1521498 exhibited slight inverse agonism.
Conclusions: Differences between GSK1521498 and naltrexone in their effects on compulsive reward seeking are arguably linked to the more selective and complete MOPr antagonism of GSK1521498 versus the partial MOPr agonism of naltrexone. GSK1521498 is also pharmacologically differentiated by its inverse agonist efficacy at high levels of MOPr expression, but this may be less likely to contribute to behavioural differentiation at patho-physiological levels of expression.
Figures
Comment in
-
Response to paper by Kelly et al "The opioid receptor pharmacology of GSK1521498 compared to other ligands with different effects on compulsive reward-related behaviors" published in Psychopharmacology 232, 305-314, 2014.Psychopharmacology (Berl). 2015 Apr;232(8):1493-4. doi: 10.1007/s00213-015-3892-3. Epub 2015 Mar 6. Psychopharmacology (Berl). 2015. PMID: 25740344 No abstract available.
-
Reply to Wang and Sadée.Psychopharmacology (Berl). 2015 Apr;232(8):1495-6. doi: 10.1007/s00213-015-3900-7. Epub 2015 Mar 11. Psychopharmacology (Berl). 2015. PMID: 25757674 No abstract available.
References
-
- Bourassa P, Bagheri H, Pineyro G, Grandbois M, Gendron L (2014) Label-free monitoring of μ opioid receptor-mediated signaling. Mol Pharmacol - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

