Ethics of iPSC-based clinical research for age-related macular degeneration: patient-centered risk-benefit analysis
- PMID: 24974102
- PMCID: PMC4225048
- DOI: 10.1007/s12015-014-9536-x
Ethics of iPSC-based clinical research for age-related macular degeneration: patient-centered risk-benefit analysis
Abstract
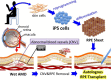
The opportunity to undergo an induced pluripotent stem cell-based autologous transplant can strike patients as a chance for a cure from a debilitating condition with few options for respite. However, when clinical studies of this caliber present themselves, patients and researchers, each with their own set of motives, may find it difficult to take a balanced approach to evaluating them. We present a patient-centered risk-benefit analysis of the iPSC-based clinical research currently underway in Japan, including a survey of in vitro and in vivo tests that support this project, an in-depth discussion of risks, and further elucidation of considerations patients may wish to consider. The arguments presented will assist patients in undertaking a more informed decision-making process.
Figures


Similar articles
-
Induced pluripotent stem cell-based therapy for age-related macular degeneration.Expert Opin Biol Ther. 2017 Sep;17(9):1113-1126. doi: 10.1080/14712598.2017.1346079. Epub 2017 Jun 30. Expert Opin Biol Ther. 2017. PMID: 28664762 Review.
-
[Development of Cellular and Tissue-based Products for Retinal Regenerative Medicine].Yakugaku Zasshi. 2017;137(1):23-29. doi: 10.1248/yakushi.16-00225. Yakugaku Zasshi. 2017. PMID: 28049891 Review. Japanese.
-
Tumorigenicity studies of induced pluripotent stem cell (iPSC)-derived retinal pigment epithelium (RPE) for the treatment of age-related macular degeneration.PLoS One. 2014 Jan 14;9(1):e85336. doi: 10.1371/journal.pone.0085336. eCollection 2014. PLoS One. 2014. PMID: 24454843 Free PMC article.
-
An eye to the future: Researchers debate best path for stem cell-derived therapies.Nat Med. 2016 Feb;22(2):116-9. doi: 10.1038/nm0216-116. Nat Med. 2016. PMID: 26845400 No abstract available.
-
Autologous stem cell therapy for inherited and acquired retinal disease.Regen Med. 2018 Jan;13(1):89-96. doi: 10.2217/rme-2017-0089. Epub 2018 Jan 23. Regen Med. 2018. PMID: 29360008 Free PMC article. Review.
Cited by
-
Cellular Reparative Mechanisms of Mesenchymal Stem Cells for Retinal Diseases.Int J Mol Sci. 2017 Jul 28;18(8):1406. doi: 10.3390/ijms18081406. Int J Mol Sci. 2017. PMID: 28788088 Free PMC article. Review.
-
Tapping Stem Cells to Target AMD: Challenges and Prospects.J Clin Med. 2015 Jan 29;4(2):282-303. doi: 10.3390/jcm4020282. J Clin Med. 2015. PMID: 26239128 Free PMC article. Review.
-
3D Bioprinting and the Future of Surgery.Front Surg. 2020 Nov 27;7:609836. doi: 10.3389/fsurg.2020.609836. eCollection 2020. Front Surg. 2020. PMID: 33330613 Free PMC article. Review.
-
Efficient derivation and inducible differentiation of expandable skeletal myogenic cells from human ES and patient-specific iPS cells.Nat Protoc. 2015 Jul;10(7):941-58. doi: 10.1038/nprot.2015.057. Epub 2015 Jun 4. Nat Protoc. 2015. PMID: 26042384
-
Pluripotent Stem Cells and Other Innovative Strategies for the Treatment of Ocular Surface Diseases.Stem Cell Rev Rep. 2016 Apr;12(2):171-8. doi: 10.1007/s12015-016-9643-y. Stem Cell Rev Rep. 2016. PMID: 26779895 Review.
References
-
- RIKEN (2013). Press release. http://www.riken.jp/en/pr/press/2013/20130730_1/. Accessed 24 Apr 2014.
-
- RIKEN and Foundation for Biomedical Research and Innovation (2013). Pilot safety study of iPSC-based intervention for wet-type AMD. Available at http://www.riken-ibri.jp/AMD/english/index.html, in Japanese at http://www.riken-ibri.jp/AMD/research/index.html. Accessed 24 Apr 2014.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

