Reactive oxygen species in normal and tumor stem cells
- PMID: 24974178
- PMCID: PMC4207279
- DOI: 10.1016/B978-0-12-420117-0.00001-3
Reactive oxygen species in normal and tumor stem cells
Abstract
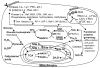
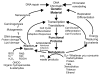
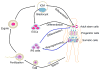
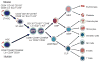
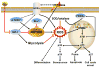
Reactive oxygen species (ROS) play an important role in determining the fate of normal stem cells. Low levels of ROS are required for stem cells to maintain quiescence and self-renewal. Increases in ROS production cause stem cell proliferation/differentiation, senescence, and apoptosis in a dose-dependent manner, leading to their exhaustion. Therefore, the production of ROS in stem cells is tightly regulated to ensure that they have the ability to maintain tissue homeostasis and repair damaged tissues for the life span of an organism. In this chapter, we discuss how the production of ROS in normal stem cells is regulated by various intrinsic and extrinsic factors and how the fate of these cells is altered by the dysregulation of ROS production under various pathological conditions. In addition, the implications of the aberrant production of ROS by tumor stem cells for tumor progression and treatment are also discussed.
Keywords: Adult stem cells; Apoptosis; Differentiation; Embryonic stem cells; Hematopoietic stem cells; Hypoxia; Ionizing radiation; Reactive oxygen species; Senescence; Tumor stem cells.
© 2014 Elsevier Inc. All rights reserved.
Figures
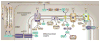

References
-
- Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, et al. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. Journal of Biological Chemistry. 2005;280:4254–4263. - PubMed
-
- Allsopp RC, Morin GB, DePinho R, Harley CB, Weissman IL. Telomerase is required to slow telomere shortening and extend replicative lifespan of HSCs during serial transplantation. Blood. 2003;102:517–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

