Gamma-glutamyl transpeptidase: redox regulation and drug resistance
- PMID: 24974180
- PMCID: PMC4388159
- DOI: 10.1016/B978-0-12-420117-0.00003-7
Gamma-glutamyl transpeptidase: redox regulation and drug resistance
Abstract
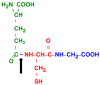
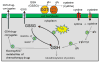
The expression of gamma-glutamyl transpeptidase (GGT) is essential to maintaining cysteine levels in the body. GGT is a cell surface enzyme that hydrolyzes the gamma-glutamyl bond of extracellular reduced and oxidized glutathione, initiating their cleavage into glutamate, cysteine (cystine), and glycine. GGT is normally expressed on the apical surface of ducts and glands, salvaging the amino acids from glutathione in the ductal fluids. GGT in tumors is expressed over the entire cell membrane and provides tumors with access to additional cysteine and cystine from reduced and oxidized glutathione in the blood and interstitial fluid. Cysteine is rate-limiting for glutathione synthesis in cells under oxidative stress. The induction of GGT is observed in tumors with elevated levels of intracellular glutathione. Studies in models of hepatocarcinogenesis show that GGT expression in foci of preneoplastic hepatocytes provides a selective advantage to the cells during tumor promotion with agents that deplete intracellular glutathione. Similarly, expression of GGT in tumors enables cells to maintain elevated levels of intracellular glutathione and to rapidly replenish glutathione during treatment with prooxidant anticancer therapy. In the clinic, the expression of GGT in tumors is correlated with drug resistance. The inhibitors of GGT block GGT-positive tumors from accessing the cysteine in extracellular glutathione. They also inhibit GGT activity in the kidney, which results in the excretion of GSH in the urine and a rapid decrease in blood cysteine levels, leading to depletion of intracellular GSH in both GGT-positive and GGT-negative tumors. GGT inhibitors are being developed for clinical use to sensitize tumors to chemotherapy.
Keywords: Cysteine; Gamma-glutamyl transferase; Gamma-glutamyl transpeptidase; Glutathione.
© 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Ahluwalia GS, Grem JL, Hao Z, Cooney DA. Metabolism and action of amino acid analog anti-cancer agents. Pharmacol Ther. 1990;46(2):243–271. - PubMed
-
- Ahmad S, Okine L, Wood R, Aljian J, Vistica DT. gamma-Glutamyl transpeptidase (gamma-GT) and maintenance of thiol pools in tumor cells resistant to alkylating agents. J Cell Physiol. 1987;131(2):240–246. - PubMed
-
- Anders MW, Dekant W. Glutathione-dependent bioactivation of haloalkenes. Annu Rev Pharmacol Toxicol. 1998;38:501–537. - PubMed
-
- Anderson ME, Bridges RJ, Meister A. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1980;96(2):848–853. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

