A comparison of reversible versus irreversible protein glutathionylation
- PMID: 24974182
- PMCID: PMC4603650
- DOI: 10.1016/B978-0-12-420117-0.00005-0
A comparison of reversible versus irreversible protein glutathionylation
Abstract
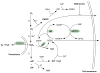
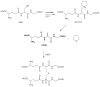
Glutathionylation is generally a reversible posttranslational modification that occurs to cysteine residues that have been exposed to reactive oxygen species (P-SSG). This cyclical process can regulate various clusters of proteins, including those involved in critical cellular signaling functions. However, certain conditions can favor the formation of dehydroamino acids, such as 2,3-didehydroalanine (2,3-dehydroalanine, DHA) and 2,3-didehydrobutyrine (2,3-dehydrobutyrine), which can act as Michael acceptors. In turn, these can form Michael adducts with glutathione (GSH), resulting in the formation of a stable thioether conjugate, an irreversible process referred to as nonreducible glutathionylation. This is predicted to be prevalent in nature, particularly in more slowly turning over proteins. Such nonreducible glutathionylation can be distinguished from the more facile cycling signaling processes and is predicted to be of gerontological, toxicological, pharmacological, and oncological relevance. Here, we compare reversible and irreversible glutathionylation.
Keywords: Dehydroalanine; Glutaredoxin; Glutathione; Glutathione S-transferases; Glutathione disulfide; Irreversible protein glutathionylation; Lanthionine; Reversible glutathionylation; γ-Glutamyl-L-cysteine.
© 2014 Elsevier Inc. All rights reserved.
Figures



References
-
- Abbas K, Riquier S, Drapier JC. Peroxiredoxins and sulfiredoxin at the crossroads of the NO and H2O2 signaling pathways. Methods in Enzymology. 2013;527:113–128. - PubMed
-
- Adams B, Lowpetch K, Thorndycroft F, Whyte SM, Young DW. Stereochemistry of reactions of the inhibitor/substrates L- and D-beta-chloroalanine with beta-mercaptoethanol catalysed by L-aspartate aminotransferase and D-amino acid aminotransferase respectively. Organic and Biomolecular Chemistry. 2005;3:3357–3364. - PubMed
-
- Asaduzzaman SM, Sonomoto K. Lantibiotics: Diverse activities and unique modes of action. Journal of Bioscience and Bioengineering. 2009;107:475–487. - PubMed
-
- Buc-Calderon P, Sipe HJ, Jr, Flitter W, Mason RP, Roberfroid M. N-acyl dehydroalanines scavenge oxygen radicals and inhibit in vitro free radical mediated processes. Chemico-Biological Interactions. 1990;73:77–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

