The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients
- PMID: 24974886
- PMCID: PMC4264911
- DOI: 10.1111/imm.12351
The effect of mammalian target of rapamycin inhibition on T helper type 17 and regulatory T cell differentiation in vitro and in vivo in kidney transplant recipients
Abstract
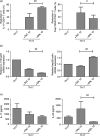
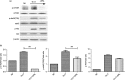
Sirolimus (SRL) is a promising alternative to calcineurin inhibitors, such as tacrolimus (TAC), in kidney transplant recipients (KTRs), but the immunological benefits of conversion from calcineurin inhibitors to SRL are not fully investigated. In the present study, we evaluated the effect of conversion from TAC to SRL on the T helper type 17/regulatory T (Th17/Treg) axis in three separate studies. First, the effect of SRL on the Th17/Treg axis was evaluated in vitro using peripheral blood mononuclear cells (PBMCs). Second, the effect of conversion from TAC to SRL on the Th17/Treg axis was studied in KTRs. Finally, the effect of SRL on CD8(+) Treg cells was evaluated. In vitro analysis of PBMCs isolated from KTRs showed that SRL suppressed Th17 cell differentiation but TAC did not. Conversion from TAC to SRL markedly decreased the number of effector memory CD8(+) T cells and significantly increased the proportion of CD4(+) and CD8(+) Treg cells compared with TAC in KTRs. SRL treatment induced the CD8(+) Treg cells, and these cells inhibited the proliferation of allogeneic CD4(+) T cells and Th17 cells. In conclusion, conversion from TAC to SRL favourably regulates Th17 and Treg cell differentiation in KTRs. These findings provide a rationale for conversion from TAC to SRL in KTRs.
Keywords: T cells; cytokines; mTOR; regulatory T cells; transplantation.
© 2014 John Wiley & Sons Ltd.
Figures




References
-
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–29. - PubMed
-
- Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605–12. - PubMed
-
- Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506–14. - PubMed
-
- Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–9. - PubMed
-
- Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

