Getting Syk: spleen tyrosine kinase as a therapeutic target
- PMID: 24975478
- PMCID: PMC4119858
- DOI: 10.1016/j.tips.2014.05.007
Getting Syk: spleen tyrosine kinase as a therapeutic target
Abstract
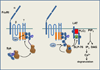
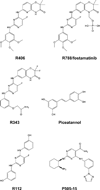
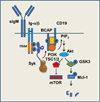
Spleen tyrosine kinase (Syk) is a cytoplasmic protein tyrosine kinase well known for its ability to couple immune cell receptors to intracellular signaling pathways that regulate cellular responses to extracellular antigens and antigen-immunoglobulin (Ig) complexes of particular importance to the initiation of inflammatory responses. Thus, Syk is an attractive target for therapeutic kinase inhibitors designed to ameliorate the symptoms and consequences of acute and chronic inflammation. Its more recently recognized role as a promoter of cell survival in numerous cancer cell types ranging from leukemia to retinoblastoma has attracted considerable interest as a target for a new generation of anticancer drugs. This review discusses the biological processes in which Syk participates that have made this kinase such a compelling drug target.
Keywords: allergic asthma; fostamatinib; leukemia; protein kinase inhibitor; rheumatoid arthritis; tyrosine phosphorylation.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Spleen tyrosine kinase (Syk) inhibitor fostamatinib limits tissue damage and fibrosis in a bleomycin-induced scleroderma mouse model.Clin Exp Rheumatol. 2015 Jul-Aug;33(4 Suppl 91):S15-22. Epub 2015 Jul 6. Clin Exp Rheumatol. 2015. PMID: 26148346
-
[Involvement of Syk in pathology of systemic autoimmune disease].Nihon Rinsho Meneki Gakkai Kaishi. 2012;35(1):56-61. doi: 10.2177/jsci.35.56. Nihon Rinsho Meneki Gakkai Kaishi. 2012. PMID: 22374444 Review. Japanese.
-
Spleen tyrosine kinases: biology, therapeutic targets and drugs.Drug Discov Today. 2010 Jul;15(13-14):517-30. doi: 10.1016/j.drudis.2010.05.001. Epub 2010 May 27. Drug Discov Today. 2010. PMID: 20553955 Review.
-
Spleen tyrosine kinase as a molecular target for treatment of leukemias and lymphomas.Expert Rev Anticancer Ther. 2010 Sep;10(9):1407-18. doi: 10.1586/era.10.112. Expert Rev Anticancer Ther. 2010. PMID: 20836676 Review.
-
Specific inhibition of spleen tyrosine kinase suppresses leukocyte immune function and inflammation in animal models of rheumatoid arthritis.J Pharmacol Exp Ther. 2012 Feb;340(2):350-9. doi: 10.1124/jpet.111.188441. Epub 2011 Oct 31. J Pharmacol Exp Ther. 2012. PMID: 22040680
Cited by
-
Transcriptomics-Based Drug Repurposing Approach Identifies Novel Drugs against Sorafenib-Resistant Hepatocellular Carcinoma.Cancers (Basel). 2020 Sep 23;12(10):2730. doi: 10.3390/cancers12102730. Cancers (Basel). 2020. PMID: 32977582 Free PMC article.
-
The dualistic role of Lyn tyrosine kinase in immune cell signaling: implications for systemic lupus erythematosus.Front Immunol. 2024 Jun 28;15:1395427. doi: 10.3389/fimmu.2024.1395427. eCollection 2024. Front Immunol. 2024. PMID: 39007135 Free PMC article. Review.
-
Genome-Wide RNAi Screening Identifies Novel Pathways/Genes Involved in Oxidative Stress and Repurposable Drugs to Preserve Cystic Fibrosis Airway Epithelial Cell Integrity.Antioxidants (Basel). 2021 Dec 2;10(12):1936. doi: 10.3390/antiox10121936. Antioxidants (Basel). 2021. PMID: 34943039 Free PMC article.
-
Inhibition of Spleen Tyrosine Kinase Restores Glucocorticoid Sensitivity to Improve Steroid-Resistant Asthma.Front Pharmacol. 2022 May 5;13:885053. doi: 10.3389/fphar.2022.885053. eCollection 2022. Front Pharmacol. 2022. PMID: 35600871 Free PMC article.
-
The Expression and Clinical Significance of Spleen Tyrosine Kinase in Patients with Coronary Heart Disease.Med Sci Monit. 2019 Mar 22;25:2112-2121. doi: 10.12659/MSM.913543. Med Sci Monit. 2019. PMID: 30898992 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

