Can consultation skills training change doctors' behaviour to increase involvement of patients in making decisions about standard treatment and clinical trials: a randomized controlled trial
- PMID: 24975503
- PMCID: PMC5810732
- DOI: 10.1111/hex.12229
Can consultation skills training change doctors' behaviour to increase involvement of patients in making decisions about standard treatment and clinical trials: a randomized controlled trial
Abstract
Background: Informed consent is required for both standard cancer treatments and experimental cancer treatments in a clinical trial. Effective and sensitive physician-patient communication about informed consent is difficult to achieve. Our aim was to train doctors in clear, collaborative and ethical communication about informed consent and evaluate the impact of training on doctor behaviour, stress and satisfaction.
Participants and methods: Participants were 21 oncologists from 10 Australian/New Zealand (ANZ) centres and 41 oncologists from 10 Swiss/German/Austrian (SGA) centres. Oncologists were randomized to participate in a 1-day workshop or not. Patients were recruited before and after the training. Doctors were asked to submit 1-2 audiotaped consultations before and after training. Doctors completed outcome measures before and after completing the post-training cohort recruitment.
Results: Ninety-five consultation interactions were audiotaped. Doctors strongly endorsed the training. ANZ intervention doctors demonstrated a significant increase in collaborative communication (P = 0.03). There was no effect of training on other doctor behaviours. Trained doctors did not demonstrate reduced stress and burnout. Patient outcomes are presented elsewhere.
Conclusions: Training can improve some aspects of the process of obtaining informed consent. Methods to increase the impact of training are required and may include longer training and more intensive follow-up.
Keywords: clinical trials; consultation skills training; decision making; oncology; physician behaviour; randomized controlled trial.
© 2014 John Wiley & Sons Ltd.
Figures
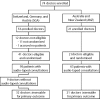

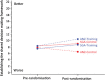
Note that Figure 3 does not take into consideration the effect of multiple patients per doctor.

References
-
- Breitsameter C. Medical decision‐making and communication of risks: an ethical perspective. Journal of Medical Ethics, 2010; 36: 349–352. - PubMed
-
- Charles C, Gafni A. Shared treatment decision making and the use of decision aids In: Kissane D, Bultz BD, Butow PN, Finlay IG. (eds) Handbook of Communication in Oncology and Palliative Care. New York: Oxford, 2010: 41–50.
-
- Siminoff LA. The ethics of communication in cancer and palliative care In: Kissane D, Bultz BD, Butow PN, Finlay IG. (eds) Handbook of Communication in Oncology and Palliative Care. New York: Oxford University Press, 2010: 51–61.
-
- Brown RF, Butow PN, Wilson‐Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting breast cancer patients' decision making preferences in oncology consultations: impact on decision related outcomes. Journal of Clinical Oncology, 2012; 30: 857–862. - PubMed
-
- Fallowfield L, Hall A, Macguire GP, Baum A, A'Hern RP. A question of choice; Results of a prospective 3 year follow up study of women with breast cancer. The Breast, 1994; 3: 202–208.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

