A prospective, randomized study on hepatotoxicity of anastrozole compared with tamoxifen in women with breast cancer
- PMID: 24975596
- PMCID: PMC4462391
- DOI: 10.1111/cas.12474
A prospective, randomized study on hepatotoxicity of anastrozole compared with tamoxifen in women with breast cancer
Abstract
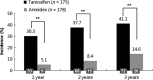
Tamoxifen and anastrozole are widely used as adjuvant treatment for early stage breast cancer, but their hepatotoxicity is not fully defined. We aimed to compare hepatotoxicity of anastrozole with tamoxifen in the adjuvant setting in postmenopausal breast cancer patients. Three hundred and fifty-three Chinese postmenopausal women with hormone receptor-positive early breast cancer were randomized to anastrozole or tamoxifen after optimal primary therapy. The primary end-point was fatty liver disease, defined as a liver-spleen ratio <0.9 as determined using a computed tomography scan. The secondary end-points included abnormal liver function and treatment failure during the 3-year follow up. The cumulative incidence of fatty liver disease after 3 years was lower in the anastrozole arm than that of tamoxifen (14.6% vs 41.1%, P < 0.0001; relative risk, 0.30; 95% CI, 0.21-0.45). However, there was no difference in the cumulative incidence of abnormal liver function (24.6% vs 24.7%, P = 0.61). Interestingly, a higher treatment failure rate was observed in the tamoxifen arm compared with anastrozole and median times to treatment failure were 15.1 months and 37.1 months, respectively (P < 0.0001; HR, 0.27; 95% CI, 0.20-0.37). The most commonly reported adverse events were 'reproductive system disorders' in the tamoxifen group (17.1%), and 'musculoskeletal disorders' in the anastrozole group (14.6%). Postmenopausal women with hormone receptor-positive breast cancer receiving adjuvant anastrozole displayed less fatty liver disease, suggesting that this drug had a more favorable hepatic safety profile than tamoxifen and may be preferred for patients with potential hepatic dysfunction.
Keywords: Anastrozole; breast cancer; clinical trial; fatty liver disease; hepatotoxicity; tamoxifen.
© 2014 The Authors. Cancer Science published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2006. NCCN Version 1.2006.
-
- Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

