Telomere maintenance mechanisms in cancer: clinical implications
- PMID: 24975603
- PMCID: PMC4262939
- DOI: 10.2174/1381612820666140630101047
Telomere maintenance mechanisms in cancer: clinical implications
Abstract
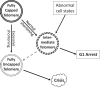
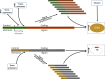
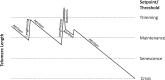
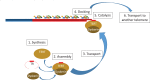
The presence of immortal cell populations with an up-regulated telomere maintenance mechanism (TMM) is an almost universal characteristic of cancers, whereas normal somatic cells are unable to prevent proliferation-associated telomere shortening and have a limited proliferative potential. TMMs and related aspects of telomere structure and function therefore appear to be ideal targets for the development of anticancer therapeutics. Such treatments would be targeted to a specific cancer-related molecular abnormality, and also be broad-spectrum in that they would be expected to be potentially applicable to most cancers. However, the telomere biology of normal and malignant human cells is a relatively young research field with large numbers of unanswered questions, so the optimal design of TMM-targeted therapeutic approaches remains unclear. This review outlines the opportunities and challenges presented by telomeres and TMMs for clinical management of cancer.
Figures



References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25: 585–621. - PubMed
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37: 614–36. - PubMed
-
- Reddel RR. The role of senescence and immortalization in carcinogenesis. Carcinogenesis. 2000;21: 477–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

