Performance characteristics of qualified cell lines for isolation and propagation of influenza viruses for vaccine manufacturing
- PMID: 24975811
- PMCID: PMC5915289
- DOI: 10.1016/j.vaccine.2014.06.045
Performance characteristics of qualified cell lines for isolation and propagation of influenza viruses for vaccine manufacturing
Abstract
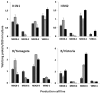
Cell culture is now available as a method for the production of influenza vaccines in addition to eggs. In accordance with currently accepted practice, viruses recommended as candidates for vaccine manufacture are isolated and propagated exclusively in hens' eggs prior to distribution to manufacturers. Candidate vaccine viruses isolated in cell culture are not available to support vaccine manufacturing in mammalian cell bioreactors so egg-derived viruses have to be used. Recently influenza A (H3N2) viruses have been difficult to isolate directly in eggs. As mitigation against this difficulty, and the possibility of no suitable egg-isolated candidate viruses being available, it is proposed to consider using mammalian cell lines for primary isolation of influenza viruses as candidates for vaccine production in egg and cell platforms. To investigate this possibility, we tested the antigenic stability of viruses isolated and propagated in cell lines qualified for influenza vaccine manufacture and subsequently investigated antigen yields of such viruses in these cell lines at pilot-scale. Twenty influenza A and B-positive, original clinical specimens were inoculated in three MDCK cell lines. The antigenicity of recovered viruses was tested by hemagglutination inhibition using ferret sera against contemporary vaccine viruses and the amino acid sequences of the hemagglutinin and neuraminidase were determined. MDCK cell lines proved to be highly sensitive for virus isolation. Compared to the virus sequenced from the original specimen, viruses passaged three times in the MDCK lines showed up to 2 amino acid changes in the hemagglutinin. Antigenic stability was also established by hemagglutination inhibition titers comparable to those of the corresponding reference virus. Viruses isolated in any of the three MDCK lines grew reasonably well but variably in three MDCK cells and in VERO cells at pilot-scale. These results indicate that influenza viruses isolated in vaccine certified cell lines may well qualify for use in vaccine production.
Keywords: Antigenic stability; Genetic stability; Influenza vaccines; MDCK; VERO; Vaccine-certified cell lines; Virus isolation; Virus purification.
Published by Elsevier Ltd.
Conflict of interest statement
Figures
Similar articles
-
Evaluation of a qualified MDCK cell line for virus isolation to develop cell-based influenza vaccine viruses with appropriate antigenicity.Vaccine. 2024 Oct 3;42(23):126242. doi: 10.1016/j.vaccine.2024.126242. Epub 2024 Aug 29. Vaccine. 2024. PMID: 39213922
-
Comparison of immunogenicity of cell-and egg-passaged viruses for manufacturing MDCK cell culture-based influenza vaccines.Virus Res. 2015 Jun 2;204:40-6. doi: 10.1016/j.virusres.2015.04.005. Epub 2015 Apr 17. Virus Res. 2015. PMID: 25892718
-
Antigenic characterization of influenza viruses produced using synthetic DNA and novel backbones.Vaccine. 2016 Jul 12;34(32):3641-8. doi: 10.1016/j.vaccine.2016.05.031. Epub 2016 May 21. Vaccine. 2016. PMID: 27219338 Free PMC article.
-
Production and antigenic properties of influenza virus from suspension MDCK-siat7e cells in a bench-scale bioreactor.Vaccine. 2010 Oct 18;28(44):7193-201. doi: 10.1016/j.vaccine.2010.08.059. Epub 2010 Aug 26. Vaccine. 2010. PMID: 20800699
-
Cell-Based Manufacturing Technology Increases Antigenic Match of Influenza Vaccine and Results in Improved Effectiveness.Vaccines (Basel). 2022 Dec 26;11(1):52. doi: 10.3390/vaccines11010052. Vaccines (Basel). 2022. PMID: 36679895 Free PMC article. Review.
Cited by
-
Cell culture-derived influenza vaccines in the severe 2017-2018 epidemic season: a step towards improved influenza vaccine effectiveness.NPJ Vaccines. 2018 Oct 9;3:44. doi: 10.1038/s41541-018-0079-z. eCollection 2018. NPJ Vaccines. 2018. PMID: 30323955 Free PMC article. Review.
-
Developments and current challenges in the process of cell culture-based seasonal influenza vaccine manufacture in Japan.Glob Health Med. 2024 Apr 30;6(2):93-100. doi: 10.35772/ghm.2023.01070. Glob Health Med. 2024. PMID: 38690131 Free PMC article. Review.
-
Tracking changes in adaptation to suspension growth for MDCK cells: cell growth correlates with levels of metabolites, enzymes and proteins.Appl Microbiol Biotechnol. 2021 Mar;105(5):1861-1874. doi: 10.1007/s00253-021-11150-z. Epub 2021 Feb 13. Appl Microbiol Biotechnol. 2021. PMID: 33582836 Free PMC article.
-
Replication kinetics of novel swine influenza A viruses: an approach to vaccine production.Austral J Vet Sci. 2024 May;56(2):85-90. doi: 10.4206/ajvs.562.01. Epub 2024 May 22. Austral J Vet Sci. 2024. PMID: 39991269 Free PMC article.
-
Effects of egg-adaptation on receptor-binding and antigenic properties of recent influenza A (H3N2) vaccine viruses.J Gen Virol. 2016 Jun;97(6):1333-1344. doi: 10.1099/jgv.0.000457. Epub 2016 Mar 14. J Gen Virol. 2016. PMID: 26974849 Free PMC article.
References
-
- Abelin A, Colegate T, Gardner S, Hehme N, Palache A. Lessons from pandemic influenza A(H1N1): the research-based vaccine industry's perspective. Vaccine. 2011;29:1135–8. - PubMed
-
- Doroshenko A, Halperin SA. Trivalent MDCK cell culture–derived influenza vaccine Optaflu (Novartis Vaccines) Expert Rev Vaccines. 2009;8:679–88. - PubMed
-
- Barrett PN, Portsmouth D, Ehrlich HJ. Vero cell culture-derived pandemic influenza vaccines: preclinical and clinical development. Expert Rev Vaccines. 2013;12:395–413. - PubMed
-
- Minor PD, Engelhardt OG, Wood JM, Robertson JS, Blayer S, Colegate T, et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine. 2009;27:2907–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

