The inflammophilic character of the periodontitis-associated microbiota
- PMID: 24976068
- PMCID: PMC4232466
- DOI: 10.1111/omi.12065
The inflammophilic character of the periodontitis-associated microbiota
Abstract
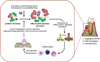
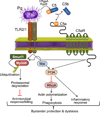
In periodontitis, dysbiotic microbial communities exhibit synergistic interactions for enhanced protection from host defenses, nutrient acquisition, and persistence in an inflammatory environment. This review discusses evidence that periodontitis-associated communities are 'inflammo-philic' (=loving or attracted to inflammation) in that they have evolved to not only endure inflammation but also to take advantage of it. In this regard, inflammation can drive the selection and enrichment of these pathogenic communities by providing a source of nutrients in the form of tissue breakdown products (e.g. degraded collagen peptides and heme-containing compounds). In contrast, those species that cannot benefit from the altered ecological conditions of the inflammatory environment, or for which host inflammation is detrimental, are likely to be outcompeted. Consistent with the concept that inflammation fosters the growth of dysbiotic microbial communities, the bacterial biomass of human periodontitis-associated biofilms was shown to increase with increasing periodontal inflammation. Conversely, anti-inflammatory treatments in animal models of periodontitis were shown to diminish the periodontal bacterial load, in addition to protecting from bone loss. The selective flourishing of inflammophilic bacteria can perpetuate inflammatory tissue destruction by setting off a 'vicious cycle' for disease progression, in which dysbiosis and inflammation reinforce each other. Therefore, the control of inflammation appears to be central to the treatment of periodontitis, as it is likely to control both dysbiosis and disease progression.
Keywords: P. gingivalis; Toll-like receptors; dysbiosis; inflammation; periodontitis.
© 2014 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Armitage GC. Classifying periodontal diseases--a long-standing dilemma. Periodontol 2000. 2002;30:9–23. - PubMed
-
- Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160:403–409. - PubMed
-
- Baker PJ, Carter S, Dixon M, Evans RT, Roopenian DC. Serum antibody response to oral infection precedes but does not prevent Porphyromonas gingivalis-induced alveolar bone loss in mice. Oral Microbiol Immunol. 1999;14:194–196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

