Distinct antifibrogenic effects of erlotinib, sunitinib and sorafenib on rat pancreatic stellate cells
- PMID: 24976727
- PMCID: PMC4069318
- DOI: 10.3748/wjg.v20.i24.7914
Distinct antifibrogenic effects of erlotinib, sunitinib and sorafenib on rat pancreatic stellate cells
Abstract
Aim: To study if three clinically available small molecule kinase inhibitors (SMI), erlotinib, sunitinib and sorafenib, exert antifibrogenic effects on pancreatic stellate cells (PSC) and analyze the basis of their action.
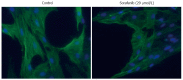
Methods: Cultured rat PSC were exposed to SMI. Cell proliferation and viability were assessed employing 5-bromo-2'-deoxyuridine incorporation assay and flow cytometry, respectively. 2-Deoxy-2-[(18)F] fluoroglucose ((18)F-FDG) uptake was measured to study metabolic activity. Exhibition of the myofibroblastic PSC phenotype was monitored by immunofluorescence analysis of α-smooth muscle actin (α-SMA) expression. Levels of mRNA were determined by real-time PCR, while protein expression and phosphorylation were analyzed by immunoblotting. Transforming growth factor-β1 (TGF-β1) levels in culture supernatants were quantified by ELISA.
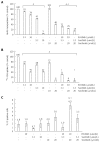
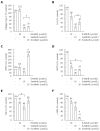
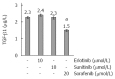
Results: All three SMI inhibited cell proliferation and (18)F-FDG uptake in a dose-dependent manner and without significant cytotoxic effects. Furthermore, additive effects of the drugs were observed. Immunoblot analysis showed that sorafenib and sunitib, but not erlotinib, efficiently blocked activation of the AKT pathway, while all three drugs displayed little effect on phosphorylation of ERK1/2. Cells treated with sorafenib or sunitinib expressed less interleukin-6 mRNA as well as less collagen type 1 mRNA and protein. Sorafenib was the only drug that also upregulated the expression of matrix metalloproteinase-2 and reduced the secretion of TGF-β1 protein. All three drugs showed insignificant or discordant effects on the mRNA and protein levels of α-SMA.
Conclusion: The tested SMI, especially sorafenib, exert inhibitory effects on activated PSC, which should be further evaluated in preclinical studies.
Keywords: Erlotinib; Fibrosis; Pancreatic stellate cell; Sorafenib; Sunitinib.
Figures


References
-
- Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421–432. - PubMed
-
- Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA, et al. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

