Cardiomyopathies: Evolution of pathogenesis concepts and potential for new therapies
- PMID: 24976920
- PMCID: PMC4072838
- DOI: 10.4330/wjc.v6.i6.478
Cardiomyopathies: Evolution of pathogenesis concepts and potential for new therapies
Abstract
Cardiomyopathies are defined as diseases of the myocardium with associated structural and functional abnormalities. Knowledge of these pathologies for a long period was not clear in clinical practice due to uncertainties regarding definition, classification and clinical diagnosis. In recent decades, major advances have been made in the understanding of the molecular and genetic issues, pathophysiology, and clinical and radiological assessment of the diseases. Progress has been made also in management of several types of cardiomyopathy. Advances in the understanding of these diseases show that cardiomyopathies represent complex entities. Here, special attention is given to evolution of classification of cardiomyopathies, with the aim of assisting clinicians to look beyond schematic diagnostic labels in order to achieve more specific diagnosis. Knowledge of the genotype of cardiomyopathies has changed the pathophysiological understanding of their etiology and clinical course, and has become more important in clinical practice for diagnosis and prevention of cardiomyopathies. New approaches for clinical and prognostic assessment are provided based on contemporary molecular mechanisms of contribution in the pathogenesis of cardiomyopathies. The genotype-phenotype complex approach for assessment improves the clinical evaluation and management strategies of these pathologies. The review covers also the important role of imaging methods, particularly echocardiography, and cardiac magnetic resonance imaging in the evaluation of different types of cardiomyopathies. In summary, this review provides complex presentation of current state of cardiomyopathies from genetics to management aspects for cardiovascular specialists.
Keywords: Arrhythmogenic cardiomyopathy; Dilated cardiomyopathy; Hypertrophic cardiomyopathy; Restrictive cardiomyopathy; Secondary cardiomyopathy.
Figures

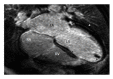
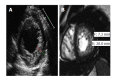


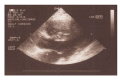

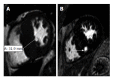

References
-
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. - PubMed
-
- Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. - PubMed
-
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752–762. - PubMed
-
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

