Manufacturing economics of plant-made biologics: case studies in therapeutic and industrial enzymes
- PMID: 24977145
- PMCID: PMC4058100
- DOI: 10.1155/2014/256135
Manufacturing economics of plant-made biologics: case studies in therapeutic and industrial enzymes
Abstract
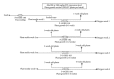
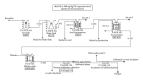
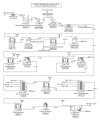
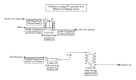
Production of recombinant biologics in plants has received considerable attention as an alternative platform to traditional microbial and animal cell culture. Industrially relevant features of plant systems include proper eukaryotic protein processing, inherent safety due to lack of adventitious agents, more facile scalability, faster production (transient systems), and potentially lower costs. Lower manufacturing cost has been widely claimed as an intuitive feature of the platform by the plant-made biologics community, even though cost information resides within a few private companies and studies accurately documenting such an advantage have been lacking. We present two technoeconomic case studies representing plant-made enzymes for diverse applications: human butyrylcholinesterase produced indoors for use as a medical countermeasure and cellulases produced in the field for the conversion of cellulosic biomass into ethanol as a fuel extender. Production economics were modeled based on results reported with the latest-generation expression technologies on Nicotiana host plants. We evaluated process unit operations and calculated bulk active and per-dose or per-unit costs using SuperPro Designer modeling software. Our analyses indicate that substantial cost advantages over alternative platforms can be achieved with plant systems, but these advantages are molecule/product-specific and depend on the relative cost-efficiencies of alternative sources of the same product.
Figures
References
-
- Pogue GP, Vojdani F, Palmer KE, et al. Production of pharmaceutical-grade recombinant aprotinin and a monoclonal antibody product using plant-based transient expression systems. Plant Biotechnology Journal. 2010;8(5):638–654. - PubMed
-
- Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL. Transient expression systems for plant-derived biopharmaceuticals. Expert Review of Vaccines. 2010;9(8):859–876. - PubMed
-
- Klimyuk V, Herz S, Butler J, Haydon H. Production of recombinant antigens and antibodies in Nicotiana benthamiana Using “Magnifection” technology: GMP-compliant facilities for small- and large-scale manufacturing. Current Topics in Microbiology and Immunology. 2014;375:127–154. - PubMed
-
- Gleba YY, Tusé D, Giritch A. Plant viral vectors for delivery by agrobacterium. Current Topics in Microbiology and Immunology. 2014;375:155–192. - PubMed

