Galectin-4 expression is associated with reduced lymph node metastasis and modulation of Wnt/β-catenin signalling in pancreatic adenocarcinoma
- PMID: 24977327
- PMCID: PMC4170638
- DOI: 10.18632/oncotarget.2104
Galectin-4 expression is associated with reduced lymph node metastasis and modulation of Wnt/β-catenin signalling in pancreatic adenocarcinoma
Abstract
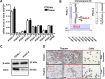
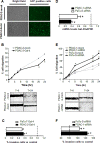
Galectin-4 (Gal-4) has been recently identified as a pivotal factor in the migratory capabilities of a set of defined pancreatic ductal adenocarcinoma (PDAC) cell lines using zebrafish as a model system. Here we evaluated the expression of Gal-4 in PDAC tissues selected according to their lymph node metastatic status (N0 vs. N1), and investigated the therapeutic potential of targeting the cross-link with the Wnt signaling pathway in primary PDAC cells. Analysis of Gal-4 expression in PDACs showed high expression of Gal-4 in 80% of patients without lymph node metastasis, whereas 70% of patients with lymph node metastases had low Gal-4 expression. Accordingly, in primary PDAC cells high Gal-4 expression was negatively associated with migratory and invasive ability in vitro and in vivo. Knockdown of Gal-4 in primary PDAC cells with high Gal-4 expression resulted in significant increase of invasion (40%) and migration (50%, P<0.05), whereas enforced expression of Gal-4 in primary cells with low Gal-4 expression reduced the migratory and invasive behavior compared to the control cells. Gal-4 markedly reduces β-catenin levels in the cell, counteracting the function of Wnt signaling, as was assessed by down-regulation of survivin and cyclin D1. Furthermore, Gal-4 sensitizes PDAC cells to the Wnt inhibitor ICG-001, which interferes with the interaction between CREB binding protein (CBP) and β-catenin. Collectively, our data suggest that Gal-4 lowers the levels of cytoplasmic β-catenin, which may lead to lowered availability of nuclear β-catenin, and consequently diminished levels of nuclear CBP-β-catenin complex and reduced activation of the Wnt target genes. Our findings provide novel insights into the role of Gal-4 in PDAC migration and invasion, and support the analysis of Gal-4 for rational targeting of Wnt/β-catenin signaling in the treatment of PDAC.
Figures




References
-
- Hidalgo M. New insights into pancreatic cancer biology. Ann Oncol. 2012;23(Suppl 10):x135–138. - PubMed
-
- Cummings RD, Liu FT. Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of Glycobiology. 2. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. Galectins. Chapter 33. - PubMed
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, et al. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76(4):597–598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

