Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm
- PMID: 24977854
- PMCID: PMC4162368
- DOI: 10.1364/OE.22.015982
Localization events-based sample drift correction for localization microscopy with redundant cross-correlation algorithm
Abstract
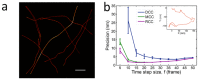
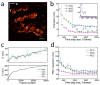
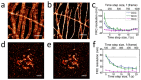
Highly accurate sample drift correction is essential in super-resolution localization microscopy to guarantee a high spatial resolution, especially when the technique is used to visualize small cell organelle. Here we present a localization events-based drift correction method using a redundant cross-correlation algorithm originally developed to correct beam-induced motion in cryo-electron microscopy. With simulated, synthesized as well as experimental data, we have demonstrated its superior precision compared to previously published localization events-based drift correction methods. The major advantage of this method is the robustness when the number of localization events is low, either because a short correction time step is required or because the imaged structure is small and sparse. This method has allowed us to improve the effective resolution when imaging Golgi apparatus in mammalian cells.
Figures

References
-
- Egner A., Geisler C., von Middendorff C., Bock H., Wenzel D., Medda R., Andresen M., Stiel A. C., Jakobs S., Eggeling C., Schönle A., Hell S. W., “Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters,” Biophys. J. 93(9), 3285–3290 (2007). 10.1529/biophysj.107.112201 - DOI - PMC - PubMed
-
- Lew M. D., Lee S. F., Ptacin J. L., Lee M. K., Twieg R. J., Shapiro L., Moerner W. E., “Three-dimensional superresolution colocalization of intracellular protein superstructures and the cell surface in live Caulobacter crescentus,” Proc. Natl. Acad. Sci. U.S.A. 108(46), E1102–E1110 (2011). 10.1073/pnas.1114444108 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

