The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice
- PMID: 24979062
- PMCID: PMC4132657
- DOI: 10.1111/mmi.12693
The Yersinia pestis siderophore, yersiniabactin, and the ZnuABC system both contribute to zinc acquisition and the development of lethal septicaemic plague in mice
Abstract
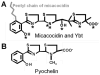
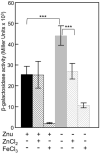
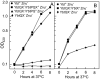
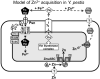
Bacterial pathogens must overcome host sequestration of zinc (Zn(2+) ), an essential micronutrient, during the infectious disease process. While the mechanisms to acquire chelated Zn(2+) by bacteria are largely undefined, many pathogens rely upon the ZnuABC family of ABC transporters. Here we show that in Yersinia pestis, irp2, a gene encoding the synthetase (HMWP2) for the siderophore yersiniabactin (Ybt) is required for growth under Zn(2+) -deficient conditions in a strain lacking ZnuABC. Moreover, growth stimulation with exogenous, purified apo-Ybt provides evidence that Ybt may serve as a zincophore for Zn(2+) acquisition. Studies with the Zn(2+) -dependent transcriptional reporter znuA::lacZ indicate that the ability to synthesize Ybt affects the levels of intracellular Zn(2+) . However, the outer membrane receptor Psn and TonB as well as the inner membrane (IM) ABC transporter YbtPQ, which are required for Fe(3+) acquisition by Ybt, are not needed for Ybt-dependent Zn(2+) uptake. In contrast, the predicted IM protein YbtX, a member of the Major Facilitator Superfamily, was essential for Ybt-dependent Zn(2+) uptake. Finally, we show that the ZnuABC system and the Ybt synthetase HMWP2, presumably by Ybt synthesis, both contribute to the development of a lethal infection in a septicaemic plague mouse model.
© 2014 John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Althaus EW, Outten CE, Olson KE, Cao H, O'Halloran TV. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6559–6569. - PubMed
-
- Ammendola S, Pasquali P, Pistoia C, Petrucci P, Petrarca P, Rotilio G, Battistoni A. High-affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to the virulence of Salmonella enterica. Infect Immun. 2007;75:5867–5876. - PMC - PubMed
-
- Andreini C, Banci L, Bertini I, Rosato A. Zinc through the three domains of life. J Proteome Res. 2006;5:3173–3178. - PubMed
-
- Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T, Durmort C. Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence. Mol Microbiol. 2011;82:904–916. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

