Phosphorylation stoichiometries of human eukaryotic initiation factors
- PMID: 24979134
- PMCID: PMC4139797
- DOI: 10.3390/ijms150711523
Phosphorylation stoichiometries of human eukaryotic initiation factors
Abstract
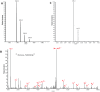
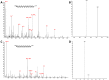
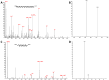
Eukaryotic translation initiation factors are the principal molecular effectors regulating the process converting nucleic acid to functional protein. Commonly referred to as eIFs (eukaryotic initiation factors), this suite of proteins is comprised of at least 25 individual subunits that function in a coordinated, regulated, manner during mRNA translation. Multiple facets of eIF regulation have yet to be elucidated; however, many of the necessary protein factors are phosphorylated. Herein, we have isolated, identified and quantified phosphosites from eIF2, eIF3, and eIF4G generated from log phase grown HeLa cell lysates. Our investigation is the first study to globally quantify eIF phosphosites and illustrates differences in abundance of phosphorylation between the residues of each factor. Thus, identification of those phosphosites that exhibit either high or low levels of phosphorylation under log phase growing conditions may aid researchers to concentrate their investigative efforts to specific phosphosites that potentially harbor important regulatory mechanisms germane to mRNA translation.
Figures
References
-
- Hinnebusch A.G. eIF3: A versatile scaffold for translation initiation complexes. Trends Biochem. Sci. 2006;31:553–562. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

