Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission
- PMID: 24979199
- PMCID: PMC4456193
- DOI: 10.1002/14651858.CD008152.pub3
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission
Update in
-
Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2018 Feb 02;2:CD008152. doi: 10.1002/14651858.CD008152.pub5. PMID: 25693791 Free PMC article. Updated.
Abstract
Background: Mosquitoes become infected with Plasmodium when they ingest gametocyte-stage parasites from an infected person's blood. Plasmodium falciparum gametocytes are sensitive to the drug primaquine (PQ) and other 8-aminoquinolines (8AQ); these drugs could prevent parasite transmission from infected people to mosquitoes, and consequently reduce the incidence of malaria. However, PQ will not directly benefit the individual, and could be harmful to those with glucose-6-phosphate dehydrogenase (G6PD) deficiency.In 2010, The World Health Organization (WHO) recommended a single dose of PQ at 0.75 mg/kg, alongside treatment for P. falciparum malaria to reduce transmission in areas approaching malaria elimination. In 2013 the WHO revised this to 0.25 mg/kg due to concerns about safety.
Objectives: To assess whether giving PQ or an alternative 8AQ alongside treatment for P. falciparum malaria reduces malaria transmission, and to estimate the frequency of severe or haematological adverse events when PQ is given for this purpose.
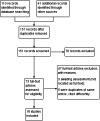
Search methods: We searched the following databases up to 10 Feb 2014 for trials: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; metaRegister of Controlled Trials (mRCT); and the WHO trials search portal using 'malaria*', 'falciparum', and 'primaquine' as search terms. In addition, we searched conference proceedings and reference lists of included studies, and contacted researchers and organizations.
Selection criteria: Randomized controlled trials (RCTs) or quasi-RCTs comparing PQ (or alternative 8AQ) given as a single dose or short course alongside treatment for P. falciparum malaria with malaria treatment given without PQ/8AQ in adults or children.
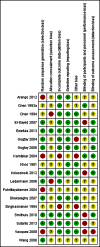
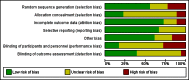
Data collection and analysis: Two authors independently screened all abstracts, applied inclusion criteria, and extracted data. We sought evidence of an impact on transmission (community incidence), infectiousness (mosquitoes infected from humans) and potential infectiousness (gametocyte measures). We calculated the area under the curve (AUC) for gametocyte density over time for comparisons for which data were available. We sought data on haematological and other adverse effects, as well as secondary outcomes of asexual clearance time and recrudescence. We stratified by whether the malaria treatment regimen included an artemisinin derivative or not; by PQ dose category (low < 0.4 mg/kg; medium ≥ 0.4 to < 0.6 mg/kg; high ≥ 0.6 mg/kg); and by PQ schedules. We used the GRADE approach to assess evidence quality.
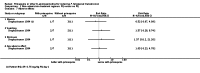
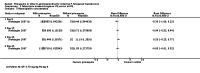
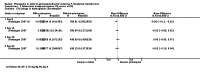
Main results: We included 17 RCTs and one quasi-RCT. Eight studies tested for G6PD status: six then excluded participants with G6PD deficiency, one included only those with G6PD deficiency, and one included all irrespective of status. The remaining ten trials either did not report on whether they tested (8), or reported that they did not test (2). Nine trials included study arms with artemisinin-based malaria treatment regimens, and eleven included study arms with non-artemisinin-based treatments.Only two trials evaluated PQ given at low doses (0.25 mg/kg in one and 0.1 mg/kg in the other). PQ with artemisinin-based treatments: No trials evaluated effects on malaria transmission directly (incidence, prevalence, or entomological inoculation rate), and none evaluated infectiousness to mosquitoes. For potential infectiousness, the proportion of people with detectable gametocytaemia on day eight was reduced by around two thirds with high dose PQ category (RR 0.29, 95% CI 0.22 to 0.37, seven trials, 1380 participants, high quality evidence), and with medium dose PQ category (RR 0.34, 95% CI 0.19 to 0.59, two trials, 269 participants, high quality evidence), but the trial evaluating low dose PQ category (0.1 mg/kg) did not demonstrate an effect (RR 0.67, 95% CI 0.44 to 1.02, one trial, 223 participants, low quality evidence). Reductions in log(10)AUC estimates for gametocytaemia on days 1 to 43 with medium and high doses ranged from 24.3% to 87.5%. For haemolysis, one trial reported percent change in mean haemoglobin against baseline, and did not detect a difference between the two arms (very low quality evidence). PQ with non-artemisinin treatments: No trials assessed effects on malaria transmission directly. Two small trials from the same laboratory evaluated infectiousness to mosquitoes, and report that infectivity was eliminated on day 8 in 15/15 patients receiving high dose PQ compared to 1/15 in the control group (low quality evidence). For potential infectiousness, the proportion of people with detectable gametocytaemia on day 8 was reduced by around half with high dose PQ category (RR 0.44, 95% CI 0.27 to 0.70, three trials, 206 participants, high quality evidence), and by around a third with medium dose category (RR 0.62, 0.50 to 0.76, two trials, 283 participants, high quality evidence), but the single trial using low dose PQ category did not demonstrate a difference between groups (one trial, 59 participants, very low quality evidence). Reduction in log(10)AUC for gametocytaemia days 1 to 43 were 24.3% and 27.1% for two arms in one trial giving medium dose PQ. No trials systematically sought evidence of haemolysis.Two trials evaluated the 8AQ bulaquine, and suggest the effects may be greater than PQ, but the small number of participants (n = 112) preclude a definite conclusion.
Authors' conclusions: In individual patients, PQ added to malaria treatments reduces gametocyte prevalence when given in doses greater than 0.4 mg/kg. Whether this translates into preventing people transmitting malaria to mosquitoes has rarely been tested in controlled trials, but there appeared to be a strong reduction in infectiousness in the two small studies that evaluated this. No included trials evaluated whether this policy has an impact on community malaria transmission either in low-endemic settings approaching elimination, or in highly-endemic settings where many people are infected but have no symptoms and are unlikely to be treated.For the currently recommended low dose regimen, there is little direct evidence to be confident that the effect of reduction in gametocyte prevalence is preserved.Most trials excluded people with G6PD deficiency, and thus there is little reliable evidence from controlled trials of the safety of PQ in single dose or short course.
Figures


















Update of
-
Primaquine for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008152. doi: 10.1002/14651858.CD008152.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. PMID: 22972117 Updated.
References
-
- Arango EA, Upegui UA, Carmona-Fonseca J. Efficacy of different primaquine-based antimalarial regimens againstPlasmodium falciparum gametocytemia. Acta Tropica. 2012;122((2012)):177–82. - PubMed
-
- Chen PQ, Li GQ, Guo XB, Fu YX, He KR, Fu LC, et al. A double blind study on the infectivity of gametocytes of P. falciparum in patients treated with mefloquine and Fansimef. Journal of Guangzhou College of Traditional Chinese Medicine. 1993;10((1)):1–5.
-
- Chen PQ, Li GQ, Guo XB. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;74((4)):209-10, 253-4. - PubMed
-
- Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;107((9)):709–11. - PubMed
-
- El-Sayed B, El-Zaki SE, Babiker H, Gadalla N, Ageep T, Mansour F, et al. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS ONE. 2007;2((12)):e1311. - PMC - PubMed
References to studies excluded from this review
-
- Baird JK, Wiady I, Sutanihardja A, Suradi, Purnomo, Basri H, et al. Short report: therapeutic efficacy of chloroquine combined with primaquine against Plasmodium falciparum in northeastern Papua, Indonesia. 2002;66(6):659–60. American Journal of Tropical Medicine and Hygiene. - PubMed
-
- Barber MA, Komp WHW, Newman BM. The effect of small doses of plasmochin on the viability of gametocytes of malaria as measured by mosquito infection experiments. Public Health Reports. 1929;44((24)):1409–20.
-
- Barber MA, Rice JB, Brown JY. Malaria studies on the Firestone Rubber Plantation in Liberia, West Africa. American Journal of Hygiene. 1932;15((3)):601–33.
-
- Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. American Journal of Tropical Medicine & Hygiene. 1998;58((5)):645–9. - PubMed
-
- Bunnag D, Harinasuta T, Pinichpongse D, Suntharasamai P. Effect of primaquine on gametocytes of Plasmodium falciparum in Thailand. Lancet. 1980;2((8185)):91. - PubMed
References to studies awaiting assessment
-
- Chen L. Efficacy of artemether/primaquine against drug resistant P. falciparum. Journal of Applied Medicine. 1993;1((1)):31–3. [Chinese]
-
- Ishii A, Ohta N, Owhashi M, Kawabata M, Chung D, Bobogare A, et al. Trials of transmission blocking of P. falciparum with single dose primaquine in villages of Solomon Islands. MIM conference October 2009 MIM 16723361.
-
- Li J, et al. Artemether combined with primaquine for treatment of 50 Pf cases. Journal of Applied Medicine. 2006;22((19)):2299–300. [Chinese]
References to ongoing studies
-
- Primaquine's Gametocytocidal Efficacy in Malaria Asymptomatic Carriers Treated With Dihydroartemisinin-piperaquine in The Gambia. Ongoing study August 2013; December 2014 (final data collection date for primary outcome measure)
-
- Phase 2a Dose Escalation Study of the Efficacy, Safety, and Pharmacokinetics of Low Dose Primaquine for Gametocytocidal Activity Against P. Falciparum in Sub-Saharan Africa and South East Asia. Ongoing study September 2014 (final data collection date for primary outcome measure)
-
- Surveillance and Treatment With Dihydroartemisinin-piperaquine Plus Primaquine (MTC Belu)Sub-title: Impact of Mass Screening and Selective Treatment With Dihydroartemisinin-piperaquine Plus Primaquine on Malaria Transmission in High Endemic Area, Belu Regency, Nusa Tenggara Timur Province, Indonesia: a Randomized Cluster Trial. Ongoing study June 2013.
-
- Active Surveillance for P. falciparum Drug Resistance With Assessment of Transmission Blocking Activity of Single Dose Primaquine in Cambodia. Ongoing study December 2012; December 2014 (final data collection date for primary outcome measure)
-
- The Optimal Timing of Primaquine to Prevent Malaria Transmission After Artemisinin-Combination Therapy. Ongoing study May 2013; October 2013 (final data collection date for primary outcome measure)
Additional references
-
- Arnold J, Alving AS, Hockwald RS, Clayman CB, Dern RJ, Beutler E, et al. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain) Journal of Laboratory and Clinical Medicine. 1955;46((3)):391–7. - PubMed
-
- Barnes, Ki, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, et al. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. Journal of Infectious Diseases. 2008;197((11)):1605–13. - PubMed
-
- Bhasin VK, Trager W. Chapter XI: Gametocytocidal effects in vitro of primaquine and related compounds on Plasmodium falciparum. In: Wernsdorfer WH, Trigg PI, editors. Primaquine: pharmacokinetics, metabolism, toxicity and activity. UNDP/World Bank/WHO; 1984.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

