Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer
- PMID: 24979481
- PMCID: PMC10982944
- DOI: 10.1002/14651858.CD009266.pub2
Non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer
Abstract
Background: Non-steroidal antiandrogens and castration are the main therapy options for advanced stages of prostate cancer. However, debate regarding the value of these treatment options continues.
Objectives: To assess the effects of non-steroidal antiandrogen monotherapy compared with luteinising hormone-releasing hormone agonists or surgical castration monotherapy for treating advanced stages of prostate cancer.
Search methods: We searched the Cochrane Prostatic Diseases and Urologic Cancers Group Specialized Register (PROSTATE), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science with Conference Proceedings, three trial registries and abstracts from three major conferences to 23 December 2013, together with reference lists, and contacted selected experts in the field and manufacturers.
Selection criteria: We included randomised controlled trials comparing non-steroidal antiandrogen monotherapy with medical or surgical castration monotherapy for men in advanced stages of prostate cancer.
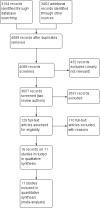
Data collection and analysis: One review author screened all titles and abstracts; only citations that were clearly irrelevant were excluded at this stage. Then, two review authors independently examined full-text reports, identified relevant studies, assessed the eligibility of studies for inclusion, assessed trial quality and extracted data. We contacted the study authors to request additional information. We used Review Manager 5 for data synthesis and used the fixed-effect model for heterogeneity less than 50%; we used the random-effects model for substantial or considerable heterogeneity.
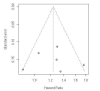
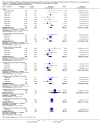
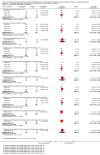
Main results: Eleven studies involving 3060 randomly assigned participants were included in this review. The quality of evidence is hampered by risk of bias. Use of non-steroidal antiandrogens decreased overall survival (hazard ratio (HR) 1.24, 95% confidence interval (CI) 1.05 to 1.48, six studies, 2712 participants) and increased clinical progression (one year: risk ratio (RR) 1.25, 95% CI 1.08 to 1.45, five studies, 2067 participants; 70 weeks: RR 1.26, 95% CI 1.08 to 1.45, six studies, 2373 participants; two years: RR 1.14, 95% CI 1.04 to 1.25, three studies, 1336 participants), as well as treatment failure (one year: RR 1.19, 95% CI 1.02 to 1.38, four studies, 1539 participants; 70 weeks: RR 1.27, 95% CI 1.05 to 1.52, five studies, 1845 participants; two years: RR 1.14, 95% CI 1.05 to 1.24, two studies, 808 participants), compared with medical or surgical castration. The quality of evidence for overall survival, clinical progression and treatment failure was rated as moderate according to GRADE. Predefined subgroup analyses showed that use of non-steroidal antiandrogens, compared with castration, was less favourable for overall survival, clinical progression (at one year, 70 weeks, two years) and treatment failure (at one year, 70 weeks, two years) in men with metastatic disease. Use of non-steroidal antiandrogens also increased the risk for treatment discontinuation due to adverse events (RR 1.82, 95% CI 1.13 to 2.94, eight studies, 1559 participants), including events such as breast pain (RR 22.97, 95% CI 14.79 to 35.67, eight studies, 2670 participants), gynaecomastia (RR 8.43, 95% CI 3.19 to 22.28, nine studies, 2774 participants) and asthenia (RR 1.77, 95% CI 1.36 to 2.31, five studies, 2073 participants). The risk of other adverse events, such as hot flashes (RR 0.23, 95% CI 0.19 to 0.27, nine studies, 2774 participants), haemorrhage (RR 0.07, 95% CI 0.01 to 0.54, two studies, 546 participants), nocturia (RR 0.38, 95% CI 0.20 to 0.69, one study, 480 participants), fatigue (RR 0.52, 95% CI 0.31 to 0.88, one study, 51 participants), loss of sexual interest (RR 0.50, 95% CI 0.30 to 0.83, one study, 51 participants) and urinary frequency (RR 0.22, 95% CI 0.11 to 0.47, one study, 480 participants) was decreased when non-steroidal antiandrogens were used. The quality of evidence for breast pain, gynaecomastia and hot flashes was rated as moderate according to GRADE. The effects of non-steroidal antiandrogens on cancer-specific survival and biochemical progression remained unclear.
Authors' conclusions: Currently available evidence suggests that use of non-steroidal antiandrogen monotherapy compared with medical or surgical castration monotherapy for advanced prostate cancer is less effective in terms of overall survival, clinical progression, treatment failure and treatment discontinuation due to adverse events. Evidence quality was rated as moderate according to GRADE. Further research is likely to have an important impact on results for patients with advanced but non-metastatic prostate cancer treated with non-steroidal antiandrogen monotherapy. However, we believe that research is likely not necessary on non-steroidal antiandrogen monotherapy for men with metastatic prostate cancer. Only high-quality, randomised controlled trials with long-term follow-up should be conducted. If further research is planned to investigate biochemical progression, studies with standardised follow-up schedules using measurements of prostate-specific antigen based on current guidelines should be conducted.
Conflict of interest statement
This review was supported by a Ferdinand Eisenberger grant of the Deutsche Gesellschaft für Urologie (German Society of Urology; grant ID KuF1/FE‐10).
Figures











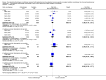














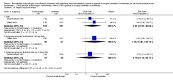

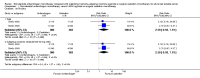


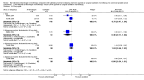




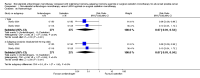











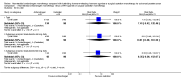


Update of
References
References to studies included in this review
Boccon‐Gibod 1997 {published data only}
-
- Boccon‐Gibod L, Fournier G, Bottet P, Marechal JM, Guiter J, Rischman P, et al. Flutamide versus orchidectomy in the treatment of metastatic prostate carcinoma. European Urology 1997;32(4):391‐5; discussion 5‐6. - PubMed
Dockery 2009 {published data only}
-
- Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Nihoyannopoulos P, Kemp M, et al. Anti‐androgens increase N‐Terminal pro‐BNP in men with prostate cancer. Clinical Endocrinology 2008;68:59‐65. - PubMed
-
- Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Rajkumar C. Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. Journal of Andrology 2009;30(4):410‐5. - PubMed
Sciarra 2004a {published data only}
-
- Sciarra A, Silverio F. Effect of nonsteroidal antiandrogen monotherapy versus castration therapy on neuroendocrine differentiation in prostate carcinoma. Urology 2004;63(3):523‐7. - PubMed
Sieber 2004 {published data only}
-
- Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. Journal of Urology 2004;171(6 Pt 1):2272‐6. - PubMed
Smith 2004 {published data only}
-
- Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. Journal of Clinical Oncology 2004;22(13):2546‐53. - PubMed
Study 0301 {published data only}
-
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. - PubMed
-
- Iversen P, Tveter K, Varenhorst E. Randomised study of casodex 50 mg monotherapy vs orchidectomy in the treatment of metastatic prostate cancer. Scandinavian Journal of Urology and Nephrology 1996;30(2):93‐8. - PubMed
Study 0302 {published data only}
-
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. - PubMed
-
- Kaisary AV, Tyrrell CJ, Beacock C, Lunglmayr G, Debruyne F. A randomized comparison of monotherapy with Casodex 50 mg daily and castration in the treatment of metastatic prostate carcinoma. European Urology 1995;28(3):215‐22. - PubMed
Study 0303 {published data only}
-
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. - PubMed
-
- Chodak G, Sharifi R, Kasimis B, Block NL, Macramalla E, Kennealey GT. Single‐agent therapy with bicalutamide: a comparison with medical or surgical castration in the treatment of advanced prostate carcinoma. Urology 1995;46(6):849‐55. - PubMed
Study 306 {published data only}
-
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150‐mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow‐up of 4 years. Urology 1998;51(3):389‐96. - PubMed
-
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Poppel H, Tammela TL, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. Journal of Urology 2000;164(5):1579‐82. - PubMed
-
- Tyrrell CJ, Blake GM, Iversen P, Kaisary AV, Melezinek I. The non‐steroidal antiandrogen, bicalutamide ('Casodex'), may preserve bone mineral density as compared with castration: results of a preliminary study. World Journal of Urology 2003;21(1):37‐42. - PubMed
-
- Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. European Urology 1998;33(5):447‐56. - PubMed
Study 307 {published data only}
-
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150‐mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow‐up of 4 years. Urology 1998;51(3):389‐96. - PubMed
-
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Poppel H, Tammela TL, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. Journal of Urology 2000;164(5):1579‐82. - PubMed
-
- Tyrrell CJ, Blake GM, Iversen P, Kaisary AV, Melezinek I. The non‐steroidal antiandrogen, bicalutamide ('Casodex'), may preserve bone mineral density as compared with castration: results of a preliminary study. World Journal of Urology 2003;21(1):37‐42. - PubMed
-
- Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. European Urology 1998;33(5):447‐56. - PubMed
Tyrrell 2006 {published data only}
-
- Tyrrell CJ, Iversen P, Tammela T, Anderson J, Bjork T, Kaisary AV, et al. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU International 2005;96(3):563‐72. Erratum in: BJU International 2006;98(3):572. - PubMed
References to studies excluded from this review
Akaza 1993 {published data only}
-
- Akaza H, Usami M, Kotake T, Koiso K, Aso Y. A randomized phase II trial of flutamide vs chlormadinone acetate in previously untreated advanced prostatic cancer. The Japan Flutamide Study Group. Japanese Journal of Clinical Oncology 1993;23(3):178‐85. - PubMed
Akaza 2003 {published data only}
-
- Akaza H, Homma Y, Okada K, Yokoyama M, Usami M, Hirao Y, et al. A prospective and randomized study of primary hormonal therapy for patients with localized or locally advanced prostate cancer unsuitable for radical prostatectomy: results of the 5‐year follow‐up. BJU International 2003;91(1):33‐6. - PubMed
Alberts 2006 {published data only}
-
- Alberts SR, Novotny PJ, Sloan JA, Danella J, Bostwick DG, Sebo TJ, et al. Flutamide in men with prostatic intraepithelial neoplasia: a randomized, placebo‐controlled chemoprevention trial. American Journal of Therapeutics 2006;13(4):291‐7. - PubMed
Aso 1993a {published data only}
-
- Aso Y, Akaza H, Koiso K, Kumamoto Y, Kawai T, Origasa S, et al. Phase I study of flutamide, a nonsteroidal antiandrogen, in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1993;39(4):381‐9. - PubMed
Aso 1993b {published data only}
-
- Aso Y, Akaza H, Kameyama S, Koiso K, Koyanagi T, Kawai T, et al. Clinical evaluation of flutamide, a pure antiandrogen, in prostatic cancer phase II dose‐finding study. Hinyokika Kiyo. Acta Urologica Japonica 1993;39(4):391‐403. - PubMed
Auvinen 2004 {published data only}
-
- Auvinen A, Hakama M, Ala‐Opas M, Vornanen T, Leppilahti M, Salminen P, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU International 2004;93(1):52‐6; discussion 6. - PubMed
Ayub 1990 {published data only}
-
- Ayub M, Levell MJ. Suppression of plasma androgens by the antiandrogen flutamide in prostatic cancer patients treated with Zoladex, a GnRH analogue. Clinical Endocrinology 1990;32(3):329‐39. - PubMed
Blackledge 1996 {published data only}
-
- Blackledge GR. High‐dose bicalutamide monotherapy for the treatment of prostate cancer. Urology 1996;47(1A Suppl):44‐7; discussion 8‐53. - PubMed
Boccardo 2002 {published data only}
-
- Boccardo F, Barichello M, Battaglia M, Carmignani G, Comeri G, Ferraris V, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer: updated results of a multicentric trial. European Urology 2002;42(5):481‐90. - PubMed
-
- Boccardo F, Rubagotti A, Borichello M, Battaglia M, Carmignani G, Comeri G, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: results of an Italian Prostate Cancer Project study. Journal of Clinical Oncology 1999;17(7):2027‐38. - PubMed
Bono 2007 {published data only}
Bruun 1996 {published data only}
-
- Bruun E, Frimodt‐Møller C. The effect of buserelin versus conventional antiandrogenic treatment in patients with T2‐4NXM1 prostatic cancer. A prospective, randomized multicentre phase III trial. Scandinavian Journal of Urology and Nephrology 1996;30(4):291‐7. - PubMed
Chang 1996 {published data only}
-
- Chang A, Yeap B, Davis T, Blum R, Hahn R, Khanna O, et al. Double‐blind, randomized study of primary hormonal treatment of stage D2 prostate carcinoma: flutamide versus diethylstilbestrol. Journal of Clinical Oncology 1996;14(8):2250‐7. - PubMed
Chatelain 1994 {published data only}
-
- Chatelain C, Rousseau V, Cosaert J. French multicentre trial comparing Casodex (ICI 176,334) monotherapy with castration plus nilutamide in metastatic prostate cancer: a preliminary report. European Urology 1994;26 Suppl 1:10‐4. - PubMed
Cockshott 1990 {published data only}
-
- Cockshott ID, Cooper KJ, Sweetmore DS, Blacklock NJ, Denis L. The pharmacokinetics of Casodex in prostate cancer patients after single and during multiple dosing. European Urology 1990;18 Suppl 3:10‐7. - PubMed
Colquhoun 2012 {published data only}
-
- Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer and Prostatic Diseases 2012;15(4):346‐52. - PubMed
Dawson 1997 {published data only}
-
- Dawson NA, Figg WD, Cooper MR, Sartor O, Bergan RC, Senderowicz AM, et al. Phase II trial of suramin, leuprolide, and flutamide in previously untreated metastatic prostate cancer. Journal of Clinical Oncology 1997;15(4):1470‐7. - PubMed
Decensi 2007 {published data only}
-
- Decensi A, Zanardi S, Puntoni M, Bandelloni R, Branchi D, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with high PSA and negative biopsy. Journal of Clinical Oncology 2007;25(18 Suppl):1500.
-
- Zanardi S, Puntoni M, Maffezzini M, Bandelloni R, Mori M, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with elevated prostate‐specific antigen and negative prostate biopsies. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(4):377‐84. - PubMed
Ekwueme 2012 {published data only}
-
- Ekwueme K, Parr N. Bicalutamide monotherapy in osteoporotic men requiring hormone manipulation for advanced prostate cancer: 12 year outcomes from a 'step up' regime. Journal of Urology 2012;187(4 Suppl):e383.
EPC program {published data only}
-
- Fourcade RO, Richaud P, Brune D, Colombel P, Sarramon JP, Fournier G, et al. Effect of bicalutamide 150 mg, after 3 years of median follow‐up, in non‐metastatic prostatic cancer [Effet du bicalutamide 150 mg, à trois ans de suivi médian, dans le cancer de la prostate non métastatique]. Progres en Urologie 2003;13(3):430‐9. - PubMed
-
- Fourcade RO, Richaud P, Coloby P, Malavaud B, Group des investigateuers français du programme EPC. Role of 150 mg bicalutamide in the treatment of prostate cancer: 3rd analysis of the EPC (early prostate cancer) program [Le bicalutamide 150 mg dans le traitement du cancer de la prostate localement avancé : des résultats à la pratique : Place du bicalutamide 150 mg dans le traitement du cancer de la prostate : hème analyse du programme EPC (Early Prostate Cancer)]. Progres en Urologie 2007;17(4 Suppl 1):891‐910. - PubMed
-
- Iversen P, Johansson J‐E, Lodding P, Lukkarinen O, Lundmo PI, Klarskov P, et al. Efficacy and tolerability of bicalutamide in early non‐metastatic prostate cancer: Latest findings from the Scandinavian Prostatic Cancer Group Study No 6 (SPCG‐6) of the early prostate cancer programme. Meeting abstract of the 21st annual European Association of Urology Congress. 2006. www.uroweb.org/index.php?id=108&AID=9715.
-
- Iversen P, Johansson JE, Lodding P, Kylmala T, Lundmo P, Klarskov P, et al. Bicalutamide 150 mg in addition to standard care for patients with early non‐metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group‐6 Study after a median follow‐up period of 7.1 years. Scandinavian Journal of Urology and Nephrology 2006;40(6):441‐52. - PubMed
-
- Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3 year median followup from the Scandinavian Prostate Cancer Group Study Number 6. Journal of Urology 2004;172(5 Pt 1):1871‐6. - PubMed
Eri 1993 {published data only}
-
- Eri LM, Tveter KJ. A prospective, placebo‐controlled study of the antiandrogen Casodex as treatment for patients with benign prostatic hyperplasia. Journal of Urology 1993;150(1):90‐4. - PubMed
Eri 2001 {published data only}
-
- Eri LM, Svindland A, Tveter KJ. The effect of bicalutamide on prostate histology. Prostate 2001;46(4):275‐80. - PubMed
Festuccia 2007 {published data only}
-
- Festuccia C, Gravina GL, Muzi P, Pomante R, Ventura L, Ricevuto E, et al. In vitro and in vivo effects of bicalutamide on the expression of TrkA and P75 neurotrophin receptors in prostate carcinoma. Prostate 2007;67(12):1255‐64. - PubMed
Gravina 2007 {published data only}
-
- Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, et al. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology 2007;70(4):728‐33. - PubMed
Henderson 2003 {published data only}
-
- Henderson A, Langley SEM, Laing RW. Is bicalutamide equivalent to goserelin for prostate volume reduction before radiation therapy? A prospective, observational study. Clinical Oncology 2003;15(6):318‐21. - PubMed
Iida 2011 {published data only}
-
- Iida K. Monotherapy versus combined androgen blockade for advanced/metastatic prostate cancer. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2011;38(13):2553‐7. - PubMed
Jacobo 1976 {published data only}
-
- Jacobo E, Schmidt JD, Weinstein SH, Flocks RH. Comparison of flutamide (SCH‐13521) and diethylstilbestrol in untreated advanced prostatic cancer. Urology 1976;8(3):231‐3. - PubMed
Johansson 1988 {published data only}
-
- Johansson J‐E, Lingårdh G, Andersson S‐O, Zador G, Beckman K‐W. Clinical evaluation of flutamide and estramustine as initial treatment of metastatic carcinoma of prostate. Urology 1987;29(1):55‐9. - PubMed
-
- Johansson JE, Andersson SO, Beckman KW, Zador G. Clinical evaluation with long‐term follow‐up of flutamide and estramustine as initial treatment of metastatic carcinoma of the prostate. American Journal of Clinical Oncology 1988;11 Suppl 2:S183‐6. - PubMed
Jones 1994 {published data only}
-
- Jones HB, Betton GR, Bowdler AL, McFarquhar RL, Middleton BJ, Lunglmayr G. Pathological and morphometric assessment of testicular parameters in patients with metastatic prostate cancer following treatment with either the antiandrogen Casodex (ZM176,334) or bilateral orchidectomy. Urological Research 1994;22(3):191‐5. - PubMed
Kaisary 1994 {published data only}
-
- Kaisary AV. Current clinical studies with a new nonsteroidal antiandrogen, Casodex. Prostate. Supplement 1994;5:27‐33. - PubMed
Kariakin 2001 {published data only}
-
- Kariakin OB, Sviridova TV, Tsodikova LB, Grishin GN, Sergeeva TN, Perekhrest MA, et al. Changes in prostate‐specific antigen in casodex (bicalutamide) monotherapy in a dose 150 mg/day given to patients with locally advanced and/or advanced prostatic cancer. Urologiia (Moscow, Russia : 1999) 2001;4:26‐9. - PubMed
Kasimis 2000 {published data only}
-
- Kasimis B, Wilding G, Kreis W, Feuerman M, Chang V, Hwang S, et al. Survival of patients who had salvage castration after failure on bicalutamide monotherapy for stage (D‐2) prostate cancer. Cancer Investigation 2000;18(7):602‐8. - PubMed
Kotake 1996a {published data only}
-
- Kotake T, Usami M, Isaka S, Shimazaki J, Oishi K, Yoshida O, et al. Phase I study of bicalutamide (Casodex), a nonsteroidal antiandrogen in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1996;42(2):143‐53. - PubMed
Kotake 1996b {published data only}
-
- Kotake T, Usami M, Isaka S, Shimazaki J, Nakano E, Okuyama A, et al. Clinical early phase II study of bicalutamide (Casodex) in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1996;42(2):157‐68. - PubMed
Kulkarni 2003 {published data only}
-
- Kulkarni JN, Gupta R. Early report of randomized double blind clinical trial of hormonal therapy of carcinoma of prostate (CAP) stage D2. Indian Journal of Urology 2003;19(2):135‐9.
Lazzaro 2007 {published data only}
-
- Lazzaro C, Bartoletti R, Guazzoni G, Orestano F, Pappagallo GL, Prezioso D, et al. Economic evaluation of different hormonal therapies for prostate cancer. Final results from the Quality of Life Antiandrogen Blockade Italian Observational Study (QuABIOS). Archivio Italiano di Urologia, Andrologia 2007;79(3):104‐7. - PubMed
Lee 2009 {published data only}
-
- Lee S, Chung YJ, Kim BH, Shim JH, Yoon SH, Shin SG, et al. Comparative pharmacokinetic evaluation of two formulations of bicalutamide 50‐mg tablets: an open‐label, randomized‐sequence, single‐dose, two‐period crossover study in healthy Korean male volunteers. Clinical Therapeutics 2009;31(12):3000‐8. - PubMed
Lee 2010 {published data only}
-
- Lee S, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Relative bioavailability and tolerability of two formulations of bicalutamide 50‐mg tablets: a randomized‐sequence, open‐label, two‐period crossover study in healthy Korean male subjects. Clinical Therapeutics 2010;32(14):2496‐501. - PubMed
Lehmusvaara 2012 {published data only}
-
- Lehmusvaara S, Erkkila T, Urbanucci A, Waltering K, Seppala J, Larjo A, et al. Chemical castration and anti‐androgens induce differential gene expression in prostate cancer. Journal of Pathology 2012;227(3):336‐45. - PubMed
Lin 2011 {published data only}
Lissoni 2002 {published data only}
-
- Lissoni P, Malugani F, Casu M, Bukovec R, Egardi R, Bordin V, et al. Effect of bicalutamide therapy on prolactin response to L‐dopa in metastatic prostate cancer patients. Neuro Endocrinology Letters 2002;23(1):61‐3. - PubMed
Loran 2001 {published data only}
-
- Loran OB, Pushkar D, Kosko D, Bernikov AN. Bicalutamide monotherapy of patients with disseminated forms of prostatic cancer. Urologiia (Moscow, Russia : 1999) 2001;3:26‐8. - PubMed
Lund 1988 {published data only}
-
- Lund F, Rasmussen F. Flutamide versus stilboestrol in the management of advanced prostatic cancer. A controlled prospective study. British Journal of Urology 1988;61(2):140‐2. - PubMed
Lunglmayr 1995 {published data only}
-
- Lunglmayr G. Efficacy and tolerability of Casodex in patients with advanced prostate cancer. Anti‐cancer Drugs 1995;6(4):508‐13. - PubMed
McGivern 2011 {published data only}
-
- McGivern U, Mitchell DM, O'Hare J, Corey G, O'Sullivan JM. How does neoadjuvant bicalutamide 150 mg monotherapy compare to luteinising hormone‐releasing hormone agonist (LHRHa) therapy in localized prostate cancer treated with radical prostatectomy? A case‐matched comparison of PSA kinetics and biochemical outcome. Journal of Clinical Oncology 2011;29(7 Suppl):146.
McGivern 2012 {published data only}
-
- McGivern U, Mitchell DM, McDowell C, O'Hare J, Corey G, O'Sullivan JM. Neoadjuvant hormone therapy for radical prostate radiotherapy: bicalutamide monotherapy vs. luteinizing hormone‐releasing hormone agonist monotherapy: a single‐institution matched‐pair analysis. Clinical Genitourinary Cancer 2012;10(3):190‐5. - PubMed
Migliari 1991 {published data only}
-
- Migliari R, Muscas G, Melis M, Garau M, Sorgia M, Scarpa M, et al. Monitoring of erection function in patients with prostatic carcinoma treated with Casodex. Archivio Italiano di Urologia, Nefrologia, Andrologia [Urological, Nephrological, and Andrological Sciences] 1991;63(1):155‐61. - PubMed
Migliari 1992 {published data only}
-
- Migliari R, Muscas G, Usai E. Effect of Casodex on sleep‐related erections in patients with advanced prostate cancer. Journal of Urology 1992;148(2 Pt 1):338‐41. - PubMed
Motofei 2011 {published data only}
-
- Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S. Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: a possible implication of androgens in male homosexual arousal. BJU International 2011;108(1):110‐5. - PubMed
Murphy 2004 {published data only}
-
- Murphy JC, Srinivas S, Terris MK. Flutamide administration at 500 mg daily has similar effects on serum testosterone to 750 mg daily. Journal of Andrology 2004;25(4):630‐4. - PubMed
Newling 1989 {published data only}
-
- Newling DW. The use of flutamide as monotherapy in the treatment of advanced prostate cancer. Progress in Clinical and Biological Research 1989;303:117‐21. - PubMed
Noldus 1996 {published data only}
-
- Noldus J, Ferrari M, Prestigicomo A, Stamey TA. Effect of flutamide and flutamide plus castration on prostate size in patients with previously untreated prostate cancer. Urology 1996;47(5):713‐8. - PubMed
Nyman 2005 {published data only}
-
- Nyman CR, Andersen JT, Lodding P, Sandin T, Varenhorst E. The patient's choice of androgen‐deprivation therapy in locally advanced prostate cancer: bicalutamide, a gonadotrophin‐releasing hormone analogue or orchidectomy. BJU International 2005;96(7):1014‐8. - PubMed
Oosterlinck 1996 {published data only}
-
- Oosterlinck W, Casselman J, Mattelaer J, Velthoven R, Kurjatkin O, Schulman C. Tolerability and safety of flutamide in monotherapy, with orchiectomy or with LHRH‐a in advanced prostate cancer patients ‐ a Belgian multicenter study of 905 patients. European Urology 1996;30(4):458‐63. - PubMed
Ostri 1991 {published data only}
-
- Ostri P, Bonnesen T, Nilsson T, Frimodt‐Moller C. Treatment of symptomatic metastatic prostatic cancer with cyproterone acetate versus orchiectomy: a prospective randomized trial. Urologia Internationalis 1991;46(2):167‐71. - PubMed
Petit 2008 {published data only}
-
- Petit JH, Gluck C, Kiger WS 3rd, Henry DL, Karasiewicz C, Talcott J, et al. Bicalutamide alone prior to brachytherapy achieves cytoreduction that is similar to luteinizing hormone‐releasing hormone analogues with less patient‐reported morbidity. Urologic Oncology 2008;26(4):372‐7. - PubMed
Prezioso 2007 {published data only}
-
- Prezioso D, Bartoletti R, Cecchi M, Cicalese V, Cunico SC, Damiano R, et al. Quality of life evaluation by the EORTC QLQ‐C30 questionnaire in patients treated with hormonal treatment in Italy. A QuABIOS group study. Archivio Italiano di Urologia, Andrologia 2007;79(3):99‐103. - PubMed
Raina 2007 {published data only}
-
- Raina R, Pahalajani G, Agarwal A, Zippe C. Long‐term effectiveness of luteinizing hormone‐releasing hormone agonist or antiandrogen monotherapy in elderly men with localized prostate cancer (T1‐2): a retrospective study. Asian Journal of Andrology 2007;9(2):253‐8. - PubMed
Raynaud 1988 {published data only}
-
- Raynaud JP. Antiandrogens in combination with LH‐RH agonists in prostate cancer. American Journal of Clinical Oncology 1988;11 Suppl 2:S132‐47. - PubMed
Scattoni 2006 {published data only}
-
- Scattoni V, Montironi R, Mazzucchelli R, Freschi M, Nava L, Losa A, et al. Pathological changes of high‐grade prostatic intraepithelial neoplasia and prostate cancer after monotherapy with bicalutamide 150 mg. BJU International 2006;98(1):54‐8. - PubMed
Scher 1997 {published data only}
-
- Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. Journal of Clinical Oncology 1997;15(8):2928‐38. - PubMed
Schröder 2000 {published data only}
Schröder 2004 {published data only}
-
- Schröder FH, Whelan P, Reijke TM, Kurth KH, Pavone‐Macaluso J, Mattelaer J, et al. Metastatic prostate cancer treated by flutamide versus cyproterone acetate. Final analysis of the "European Organization for Research and Treatment of Cancer" (EORTC) Protocol 30892. European Urology 2004;45(4):457‐64. - PubMed
Sciarra 2004b {published data only}
-
- Sciarra A, Cardi A, Silverio F. Antiandrogen monotherapy: recommendations for the treatment of prostate cancer. Urologia Internationalis 2004;72(2):91‐8. - PubMed
See 2004 {published data only}
Shipley 2011 {published data only}
-
- Shipley WU, Hunt D, Lukka HR, Major P, Heney NM, Grignon D, et al. Initial report of RTOG 9601, a phase III trial in prostate cancer: effect of anti‐androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) on freedom from progression and incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2‐3,N0 disease and elevated PSA levels. Journal of Clinical Oncology 2011;29(7 Suppl):1.
Smith 2003 {published data only}
-
- Smith MR, Fallon MA, Goode MJ. Cross‐sectional study of bone turnover during bicalutamide monotherapy for prostate cancer. Urology 2003;61(1):127‐31. - PubMed
Soloway 1996 {published data only}
-
- Soloway MS, Schellhammer PF, Smith JA Jr, Chodak GW, Vogelzang NJ, Kennealey GT. Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II non‐comparative multicenter trial evaluating safety, efficacy and long‐term endocrine effects of monotherapy. Journal of Urology 1995;154(6):2110‐4. - PubMed
-
- Soloway MS, Schellhammer PF, Smith JA, Chodak GW, Kennealey GT. Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II multicenter trial. Urology 1996;47(1 Suppl 1):33‐7; discussion 48‐53. - PubMed
Thorpe 1996 {published data only}
-
- Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O'Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. European Urology 1996;29(1):47‐54. - PubMed
Thrasher 2000 {published data only}
-
- Thrasher JB, Deeths J, Bennett C, Iyer P, Dineen MK, Zhai S, et al. Comparative study of the clinical efficacy of two dosing regimens of flutamide. Molecular Urology 2000;4(3):259‐63; discussion 265. - PubMed
Tyrrell 1998 {published data only}
-
- Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID, et al. Casodex 10‐200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. European Urology 1998;33(1):39‐53. - PubMed
Verhelst 1994 {published data only}
-
- Verhelst J, Denis L, Vliet P, Poppel H, Braeckman J, Cangh P, et al. Endocrine profiles during administration of the new non‐steroidal anti‐androgen Casodex in prostate cancer. Clinical Endocrinology 1994;41(4):525‐30. - PubMed
Wadhwa 2009 {published data only}
-
- Wadhwa VK, Weston R, Mistry R, Parr NJ. Long‐term changes in bone mineral density and predicted fracture risk in patients receiving androgen‐deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU International 2009;104(6):800‐5. - PubMed
Wadhwa 2011 {published data only}
-
- Wadhwa V, Weston R, Parr NJ. Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health‐related quality of life benefits for osteoporotic men with prostate cancer. BJU International 2011;107(12):1923‐9. - PubMed
Wirth 2004 {published data only}
-
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, et al. Prospective randomized trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for locally advanced, lymph node‐negative prostate cancer. European Urology 2004;45(3):267‐70; discussion 270. - PubMed
Yoshimura 2003 {published data only}
-
- Yoshimura K, Sumiyoshi Y, Hashimura T, Ueda T, Kamiryo Y, Yamamoto A, et al. Neoadjuvant flutamide monotherapy for locally confined prostate cancer. International Journal of Urology 2003;10(4):190‐5. - PubMed
Zanardi 2009 {published data only}
-
- Zanardi S, Puntoni M, Maffezzini M, Bandelloni R, Mori M, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with elevated prostate‐specific antigen and negative prostate biopsies. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(4):377‐84. - PubMed
Zhigang 2008 {published data only}
-
- Zhigang Z, Wenlu S. Flutamide reduced prostate cancer development and prostate stem cell antigen mRNA expression in high grade prostatic intraepithelial neoplasia. International Journal of Cancer. Journal International du Cancer 2008;122(4):864‐70. - PubMed
Additional references
Abrahamsson 2005
-
- Abrahamsson PA, Anderson J, Boccon‐Gibod L, Schulman C, Studer UE, Wirth M. Risks and benefits of hormonal manipulation as monotherapy or adjuvant treatment in localised prostate cancer. European Urology 2005;48(6):900‐5. - PubMed
Akl 2012
-
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow‐up in randomised controlled trials (LOST‐IT): systematic review. BMJ (Clinical Research Ed.) 2012;18(344):e2809. - PubMed
Als‐Nielsen 2004
-
- Als‐Nielsen B, Gluud LL, Gluud C. Methodological quality and treatment effects in randomized trials: a review of six empirical studies. Meeting abstract of the 12th Cochrane Colloquium, Ottawa (Canada). 2004. www.cochrane.org/events/colloquia.
ASCO 2004
-
- Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben‐Josef E, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen‐sensitive metastatic, recurrent, or progressive prostate cancer. Journal of Clinical Oncology 2004;22(14):2927‐41. - PubMed
ASCO 2007
-
- Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben‐Josef E, Mendelson DS, et al. Initial hormonal management of androgen‐sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. Journal of Clinical Oncology 2007;25(12):1596‐605. - PubMed
Azzouni 2012
-
- Azzouni F, Mohler J. Role of 5α‐reductase inhibitors in prostate cancer prevention and treatment. Urology 2012;79(6):1197‐205. - PubMed
Boccardo 1999
-
- Boccardo F, Rubagotti A, Barichello M, Battaglia M, Carmignani G, Comeri G, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: results of an Italian Prostate Cancer Project study. Journal of Clinical Oncology 1999;17(7):2027‐38. - PubMed
Boyle 2005
-
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Annals of Oncology 2005;16(3):481‐8. - PubMed
Chou 2010
-
- Chou R, Aronson N, Atkins D, Ismaila AS, Santaguida P, Smith DH, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health‐care program. Journal of Clinical Epidemiology 2010;63(5):502‐12. - PubMed
Daniell 1997
-
- Daniell HW. Osteoporosis after orchiectomy for prostate cancer. Journal of Urology 1997;157(2):439‐44. - PubMed
Deeks 2008
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:243‐96.
Dockery 2002
-
- Dockery F, Bulpitt CJ, Agarwal S, Rajkumar C. Testosterone suppression in men with prostate cancer is associated with increased arterial stiffness. Aging Male 2002;5(4):216‐22. - PubMed
EAU 2013
-
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD, et al. Guidelines on prostate cancer. European Association of Urology; 2013. www.uroweb.org/gls/pdf/09_Prostate_Cancer_LR.pdf (accessed 30 August 2013).
Efstathiou 2008
-
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92‐02. European Urology 2008;54(4):816‐23. - PubMed
Efstathiou 2009
Eheman 2012
Gamble 2005
-
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. - PubMed
Gartlehner 2008
-
- Gartlehner G. Assessment of adverse effects and applicability ‐ two areas not (yet) covered adequately in Cochrane reports. Zeitschrift fur Evidenz, Fortbildung und Qualitat Im Gesundheitswesen 2008;102(8):497‐502. - PubMed
Gibbs 1996
-
- Gibbs SJ, Plowman PN. Androgen deprivation and antagonism in the treatment of advanced prostatic carcinoma. Clinical Oncology 1996;8(6):346‐52. - PubMed
GLOBOCAN 2012
-
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr (accessed 8 April 2014).
Grundmark 2012
-
- Grundmark B, Garmo H, Zethelius B, Stattin P, Lambe M, Holmberg L. Anti‐androgen prescribing patterns, patient treatment adherence and influencing factors; results from the nationwide PCBaSe Sweden. European Journal of Clinical Pharmacology 2012;68(12):1619‐30. - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Gøtzsche 2006
-
- Gøtzsche PC, Hróbjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Constraints on publication rights in industry‐initiated clinical trials. JAMA 2006;295(14):1645‐6. - PubMed
Hampson 2012
-
- Hampson LA, Montie JE. Conflict of interest in urology. Journal of Urology 2012;187(6):1971‐7. - PubMed
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2004
-
- Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine 2004;23(11):1663‐82. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011c
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Huggins 2002
-
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Journal of Urology 2002;167(2 Pt 2):948‐51. - PubMed
Iversen 2002
-
- Iversen P. Antiandrogen monotherapy: indications and results. Urology 2002;60(3 Suppl 1):64‐71. - PubMed
Kunath 2012
Meerpohl 2010
-
- Meerpohl JJ, Wolff RF, Niemeyer CM, Antes G, Elm E. Editorial policies of pediatric journals: survey of instructions for authors. Archives of Pediatrics & Adolescent Medicine 2010;164(3):268‐72. - PubMed
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
Nguyen 2011
-
- Nguyen PL, Je Y, Schutz FAB, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta‐analysis of randomized trials. JAMA 2011;306(21):2359‐66. - PubMed
Okike 2007
-
- Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. Journal of Bone and Joint Surgery. American Volume 2007;89(3):608‐13. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Pildal 2007
-
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. - PubMed
Porta 2007
-
- Porta N, Bonet C, Cobo E. Discordance between reported intention‐to‐treat and per protocol analyses. Journal of Clinical Epidemiology 2007;60(7):663‐9. - PubMed
Ramm 2012
-
- Ramm O, Brubaker L. Conflicts‐of‐interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Medicine & Reconstructive Surgery 2012;18(2):79‐81. - PubMed
Review Manager 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schmitt 1999
Schulz 1996
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Seidenfeld 1999
Seidenfeld 2000
-
- Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single‐therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta‐analysis. Annals of Internal Medicine 2000;132(7):566‐77. - PubMed
Shah 2005
-
- Shah RV, Albert TJ, Bruegel‐Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine 2005;30(9):1099‐104. - PubMed
Smith 2002
-
- Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology 2002;60(3 Suppl 1):79‐85. - PubMed
Sun 2009
Sun 2012
-
- Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ (Clinical Research Ed.) 2012;344:e1553. - PubMed
Tierney 2005
-
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34(1):79‐87. - PubMed
Tyrrell 1994
-
- Tyrrell CJ. Tolerability and quality of life aspects with the anti‐androgen Casodex (ICI 176,334) as monotherapy for prostate cancer. International Casodex Investigators. European Urology 1994;26 Suppl 1:15‐9. - PubMed
Wang 2010
-
- Wang SS, Ou YC, Cheng CL, Dahm P. Evidence‐based urology in practice: when to believe a subgroup analysis?. BJU International 2010;105(2):162‐4. - PubMed
Williamson 2002
-
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. - PubMed
Wirth 2007
-
- Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. European Urology 2007;51(2):306‐14. - PubMed
Wirth 2008
-
- Wirth MP, Hakenberg OW, Froehner M. Adjuvant hormonal treatment ‐ the bicalutamide early prostate cancer program. Frontiers of Radiation Therapy and Oncology 2008;41:39‐48. - PubMed
Wood 2004
-
- Wood AM, White IR, Thompson SG. A review of published randomized controlled trials in major medical journals. Clinical Trials (London, England) 2004;1(4):368‐76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

