FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures
- PMID: 24979718
- PMCID: PMC4083475
- DOI: 10.1038/ncomms5242
FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures
Abstract
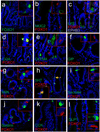
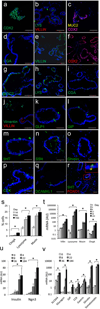
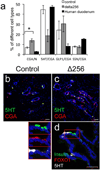
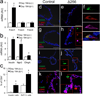
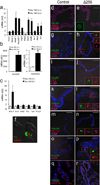
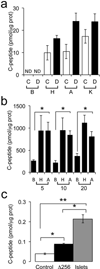
Generation of surrogate sources of insulin-producing β-cells remains a goal of diabetes therapy. While most efforts have been directed at differentiating embryonic or induced pluripotent stem (iPS) cells into β-like-cells through endodermal progenitors, we have shown that gut endocrine progenitor cells of mice can be differentiated into glucose-responsive, insulin-producing cells by ablation of transcription factor Foxo1. Here we show that FOXO1 is present in human gut endocrine progenitor and serotonin-producing cells. Using gut organoids derived from human iPS cells, we show that FOXO1 inhibition using a dominant-negative mutant or lentivirus-encoded small hairpin RNA promotes generation of insulin-positive cells that express all markers of mature pancreatic β-cells, release C-peptide in response to secretagogues and survive in vivo following transplantation into mice. The findings raise the possibility of using gut-targeted FOXO1 inhibition or gut organoids as a source of insulin-producing cells to treat human diabetes.
Figures


References
-
- Schonhoff SE, Giel-Moloney M, Leiter AB. Minireview: Development and differentiation of gut endocrine cells. Endocrinology. 2004;145:2639–2644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

