Identification of gene ontologies linked to prefrontal-hippocampal functional coupling in the human brain
- PMID: 24979789
- PMCID: PMC4084419
- DOI: 10.1073/pnas.1404082111
Identification of gene ontologies linked to prefrontal-hippocampal functional coupling in the human brain
Retraction in
-
Retraction for Dixson et al., Identification of gene ontologies linked to prefrontal-hippocampal functional coupling in the human brain.Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13582. doi: 10.1073/pnas.1415682111. Epub 2014 Sep 2. Proc Natl Acad Sci U S A. 2014. PMID: 25197092 Free PMC article. No abstract available.
Abstract
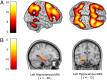
Functional interactions between the dorsolateral prefrontal cortex and hippocampus during working memory have been studied extensively as an intermediate phenotype for schizophrenia. Coupling abnormalities have been found in patients, their unaffected siblings, and carriers of common genetic variants associated with schizophrenia, but the global genetic architecture of this imaging phenotype is unclear. To achieve genome-wide hypothesis-free identification of genes and pathways associated with prefrontal-hippocampal interactions, we combined gene set enrichment analysis with whole-genome genotyping and functional magnetic resonance imaging data from 269 healthy German volunteers. We found significant enrichment of the synapse organization and biogenesis gene set. This gene set included known schizophrenia risk genes, such as neural cell adhesion molecule (NRCAM) and calcium channel, voltage-dependent, beta 2 subunit (CACNB2), as well as genes with well-defined roles in neurodevelopmental and plasticity processes that are dysfunctional in schizophrenia and have mechanistic links to prefrontal-hippocampal functional interactions. Our results demonstrate a readily generalizable approach that can be used to identify the neurogenetic basis of systems-level phenotypes. Moreover, our findings identify gene sets in which genetic variation may contribute to disease risk through altered prefrontal-hippocampal functional interactions and suggest a link to both ongoing and developmental synaptic plasticity.
Keywords: GSEA; endophenotype; functional connectivity; genetic risk variants.
Conflict of interest statement
Conflict of interest statement: A.M.-L. has received consultant fees and travel expenses from Alexza Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Defined Health, Decision Resources, Desitin Arzneimittel, Elsevier, F. Hoffmann–La Roche, Gerson Lehrman Group, Grupo Ferrer, Les Laboratoires Servier, Lilly Deutschland, Lundbeck Foundation, Outcome Sciences, Outcome Europe, PriceSpective, and Roche Pharma and has received speaker's fees from Abbott, AstraZeneca, BASF, Bristol-Myers Squibb, GlaxoSmithKline, Janssen-Cilag, Lundbeck, Pfizer Pharma, and Servier Deutschland. No other disclosures were reported.
Figures
References
-
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468(7321):194–202. - PubMed
-
- Meyer-Lindenberg AS, et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 2005;62(4):379–386. - PubMed
-
- Rasetti R, et al. Altered cortical network dynamics: A potential intermediate phenotype for schizophrenia and association with ZNF804A. Arch Gen Psychiatry. 2011;68(12):1207–1217. - PubMed
-
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660–669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

