Transcellular degradation of axonal mitochondria
- PMID: 24979790
- PMCID: PMC4084443
- DOI: 10.1073/pnas.1404651111
Transcellular degradation of axonal mitochondria
Abstract
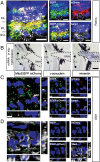
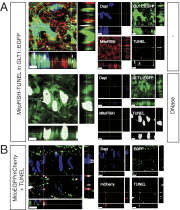
It is generally accepted that healthy cells degrade their own mitochondria. Here, we report that retinal ganglion cell axons of WT mice shed mitochondria at the optic nerve head (ONH), and that these mitochondria are internalized and degraded by adjacent astrocytes. EM demonstrates that mitochondria are shed through formation of large protrusions that originate from otherwise healthy axons. A virally introduced tandem fluorophore protein reporter of acidified mitochondria reveals that acidified axonal mitochondria originating from the retinal ganglion cell are associated with lysosomes within columns of astrocytes in the ONH. According to this reporter, a greater proportion of retinal ganglion cell mitochondria are degraded at the ONH than in the ganglion cell soma. Consistently, analyses of degrading DNA reveal extensive mtDNA degradation within the optic nerve astrocytes, some of which comes from retinal ganglion cell axons. Together, these results demonstrate that surprisingly large proportions of retinal ganglion cell axonal mitochondria are normally degraded by the astrocytes of the ONH. This transcellular degradation of mitochondria, or transmitophagy, likely occurs elsewhere in the CNS, because structurally similar accumulations of degrading mitochondria are also found along neurites in superficial layers of the cerebral cortex. Thus, the general assumption that neurons or other cells necessarily degrade their own mitochondria should be reconsidered.
Keywords: mitophagy; phagocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Neuroscience. Astrocytes eyeball axonal mitochondria.Science. 2014 Jul 25;345(6195):385-6. doi: 10.1126/science.1258295. Science. 2014. PMID: 25061193 Free PMC article. No abstract available.
-
Discovery and implications of transcellular mitophagy.Autophagy. 2014;10(12):2383-4. doi: 10.4161/15548627.2014.981920. Autophagy. 2014. PMID: 25484086 Free PMC article.
References
-
- Pankiv S, et al. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282(33):24131–24145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103412/GM/NIGMS NIH HHS/United States
- 5P41RR004050/RR/NCRR NIH HHS/United States
- 5T32GM07814/GM/NIGMS NIH HHS/United States
- R01 EY022680/EY/NEI NIH HHS/United States
- 5R01GM82949/GM/NIGMS NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- R01 GM082949/GM/NIGMS NIH HHS/United States
- R01 DA016602/DA/NIDA NIH HHS/United States
- R01 EY019960/EY/NEI NIH HHS/United States
- 5P41GM103412-25/GM/NIGMS NIH HHS/United States
- P30 NS050274/NS/NINDS NIH HHS/United States
- T32 GM007814/GM/NIGMS NIH HHS/United States
- DA016602/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

