Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles
- PMID: 24979791
- PMCID: PMC4084434
- DOI: 10.1073/pnas.1404855111
Centrosomes are autocatalytic droplets of pericentriolar material organized by centrioles
Abstract
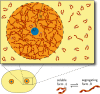
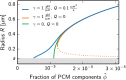
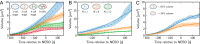
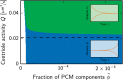
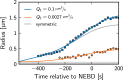
Centrosomes are highly dynamic, spherical organelles without a membrane. Their physical nature and their assembly are not understood. Using the concept of phase separation, we propose a theoretical description of centrosomes as liquid droplets. In our model, centrosome material occurs in a form soluble in the cytosol and a form that tends to undergo phase separation from the cytosol. We show that an autocatalytic chemical transition between these forms accounts for the temporal evolution observed in experiments. Interestingly, the nucleation of centrosomes can be controlled by an enzymatic activity of the centrioles, which are present at the core of all centrosomes. This nonequilibrium feature also allows for multiple stable centrosomes, a situation that is unstable in equilibrium phase separation. Our theory explains the growth dynamics of centrosomes for all cell sizes down to the eight-cell stage of the Caenorhabditis elegans embryo, and it also accounts for data acquired in experiments with aberrant numbers of centrosomes and altered cell volumes. Furthermore, the model can describe unequal centrosome sizes observed in cells with perturbed centrioles. We also propose an interpretation of the molecular details of the involved proteins in the case of C. elegans. Our example suggests a general picture of the organization of membraneless organelles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Travis J. Mysteries of the cell. How does the cell position its proteins? Science. 2011;334(6059):1048–1049. - PubMed
-
- Goehring NW, Hyman AA. Organelle growth control through limiting pools of cytoplasmic components. Curr Biol. 2012;22(9):R330–R339. - PubMed
-
- Brangwynne C. Soft active aggregates: Mechanics, dynamics and self-assembly of liquid-like intracellular protein bodies. Soft Matter. 2011;7:3052–3059.
-
- Hyman AA, Brangwynne CP. Beyond Stereospecificity: Liquids and Mesoscale Organization of Cytoplasm. Dev Cell. 2011;21(1):14–16. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

