Rag GTPases are cardioprotective by regulating lysosomal function
- PMID: 24980141
- PMCID: PMC4100214
- DOI: 10.1038/ncomms5241
Rag GTPases are cardioprotective by regulating lysosomal function
Abstract
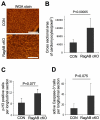
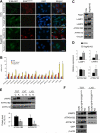
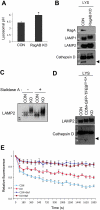
The Rag family proteins are Ras-like small GTPases that have a critical role in amino-acid-stimulated mTORC1 activation by recruiting mTORC1 to lysosome. Despite progress in the mechanistic understanding of Rag GTPases in mTORC1 activation, little is known about the physiological function of Rag GTPases in vivo. Here we show that loss of RagA and RagB (RagA/B) in cardiomyocytes results in hypertrophic cardiomyopathy and phenocopies lysosomal storage diseases, although mTORC1 activity is not substantially impaired in vivo. We demonstrate that despite upregulation of lysosomal protein expression by constitutive activation of the transcription factor EB (TFEB) in RagA/B knockout mouse embryonic fibroblasts, lysosomal acidification is compromised owing to decreased v-ATPase level in the lysosome fraction. Our study uncovers RagA/B GTPases as key regulators of lysosomal function and cardiac protection.
Figures

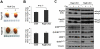





References
-
- Sekiguchi T, Hirose E, Nakashima N, Ii M, Nishimoto T. Novel G proteins, Rag C and Rag D, interact with GTP-binding proteins, Rag A and Rag B. J Biol Chem. 2001;276:7246–7257. - PubMed
-
- Dubouloz F, Deloche O, Wanke V, Cameroni E, De Virgilio C. The TOR and EGO protein complexes orchestrate microautophagy in yeast. Mol Cell. 2005;19:15–26. - PubMed
-
- Gao M, Kaiser CA. A conserved GTPase-containing complex is required for intracellular sorting of the general amino-acid permease in yeast. Nat Cell Biol. 2006;8:657–667. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

