Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells
- PMID: 24980968
- PMCID: PMC4187820
- DOI: 10.1128/IAI.01849-14
Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1α and proinflammatory cytokine secretion in cultured respiratory epithelial cells
Abstract
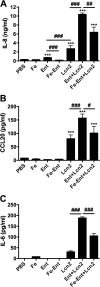
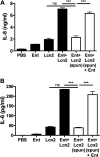
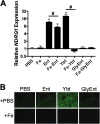
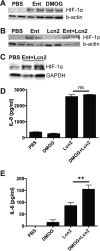
Iron is essential for many cellular processes and is required by bacteria for replication. To acquire iron from the host, pathogenic Gram-negative bacteria secrete siderophores, including enterobactin (Ent). However, Ent is bound by the host protein lipocalin 2 (Lcn2), preventing bacterial reuptake of aferric or ferric Ent. Furthermore, the combination of Ent and Lcn2 (Ent+Lcn2) leads to enhanced secretion of interleukin-8 (IL-8) compared to that induced by either stimulus alone. Modified or structurally distinct siderophores, including yersiniabactin (Ybt) and glycosylated Ent (GlyEnt, or salmochelin), deliver iron to bacteria despite the presence of Lcn2. We hypothesized that the robust immune response to Ent and Lcn2 requires iron chelation rather than the Ent+Lcn2 complex itself and also can be stimulated by Lcn2-evasive siderophores. To test this hypothesis, cultured respiratory epithelial cells were stimulated with combinations of purified siderophores and Lcn2 and analyzed by gene expression microarrays, quantitative PCR, and cytokine immunoassays. Ent caused HIF-1α protein stabilization, induced the expression of genes regulated by hypoxia-inducible factor 1α (HIF-1α), and repressed genes involved in cell cycle and DNA replication, whereas Lcn2 induced expression of proinflammatory cytokines. Iron chelation by excess Ent or Ybt significantly increased Lcn2-induced secretion of IL-8, IL-6, and CCL20. Stabilization of HIF-1α was sufficient to enhance Lcn2-induced IL-6 secretion. These data indicate that respiratory epithelial cells can respond to bacterial siderophores that evade or overwhelm Lcn2 binding by increasing proinflammatory cytokine production.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Interaction of lipocalin 2, transferrin, and siderophores determines the replicative niche of Klebsiella pneumoniae during pneumonia.mBio. 2012 Nov 20;3(6):e00224-11. doi: 10.1128/mBio.00224-11. mBio. 2012. PMID: 23169997 Free PMC article.
-
Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin.PLoS Pathog. 2009 Oct;5(10):e1000622. doi: 10.1371/journal.ppat.1000622. Epub 2009 Oct 16. PLoS Pathog. 2009. PMID: 19834550 Free PMC article.
-
Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2.Infect Immun. 2011 Aug;79(8):3309-16. doi: 10.1128/IAI.05114-11. Epub 2011 May 16. Infect Immun. 2011. PMID: 21576334 Free PMC article.
-
The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer.Biochim Biophys Acta. 2012 Aug;1826(1):129-69. doi: 10.1016/j.bbcan.2012.03.008. Epub 2012 Mar 31. Biochim Biophys Acta. 2012. PMID: 22513004 Free PMC article. Review.
-
Neutrophil gelatinase-associated lipocalin, a siderophore-binding eukaryotic protein.Biometals. 2006 Apr;19(2):211-5. doi: 10.1007/s10534-005-3251-7. Biometals. 2006. PMID: 16718606 Review.
Cited by
-
Klebsiella pneumoniae Siderophores Induce Inflammation, Bacterial Dissemination, and HIF-1α Stabilization during Pneumonia.mBio. 2016 Sep 13;7(5):e01397-16. doi: 10.1128/mBio.01397-16. mBio. 2016. PMID: 27624128 Free PMC article.
-
High Affinity Iron Acquisition Systems Facilitate but Are Not Essential for Colonization of Chickens by Salmonella Enteritidis.Front Microbiol. 2022 Mar 3;13:824052. doi: 10.3389/fmicb.2022.824052. eCollection 2022. Front Microbiol. 2022. PMID: 35308377 Free PMC article.
-
Multilocus Sequence Typing for Molecular Epidemiology of Stenotrophomonas maltophilia Clinical and Environmental Isolates from a Tertiary Hospital in West of Iran.Iran Biomed J. 2022 Mar 1;26(2):142-52. doi: 10.52547/ibj.26.2.142. Iran Biomed J. 2022. PMID: 35032967 Free PMC article.
-
Hypoxia and Innate Immunity: Keeping Up with the HIFsters.Annu Rev Immunol. 2020 Apr 26;38:341-363. doi: 10.1146/annurev-immunol-100819-121537. Epub 2020 Jan 21. Annu Rev Immunol. 2020. PMID: 31961750 Free PMC article. Review.
-
Stenotrophomonas maltophilia produces an EntC-dependent catecholate siderophore that is distinct from enterobactin.Microbiology (Reading). 2017 Nov;163(11):1590-1603. doi: 10.1099/mic.0.000545. Epub 2017 Oct 6. Microbiology (Reading). 2017. PMID: 28984234 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

