Two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways
- PMID: 24980972
- PMCID: PMC4187841
- DOI: 10.1128/IAI.01769-14
Two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways
Erratum in
-
Correction for Ma et al., two functional type VI secretion systems in avian pathogenic Escherichia coli are involved in different pathogenic pathways.Infect Immun. 2015 Aug;83(8):3340. doi: 10.1128/IAI.00615-15. Infect Immun. 2015. PMID: 26157089 Free PMC article. No abstract available.
Abstract
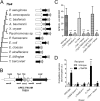
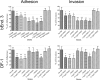
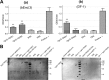
Type VI secretion systems (T6SSs) are involved in the pathogenicity of several Gram-negative bacteria. The VgrG protein, a core component and effector of T6SS, has been demonstrated to perform diverse functions. The N-terminal domain of VgrG protein is a homologue of tail fiber protein gp27 of phage T4, which performs a receptor binding function and determines the host specificity. Based on sequence analysis, we found that two putative T6SS loci exist in the genome of the avian pathogenic Escherichia coli (APEC) strain TW-XM. To assess the contribution of these two T6SSs to TW-XM pathogenesis, the crucial clpV clusters of these two T6SS loci and their vgrG genes were deleted to generate a series of mutants. Consequently, T6SS1-associated mutants presented diminished adherence to and invasion of several host cell lines cultured in vitro, decreased pathogenicity in duck and mouse infection models in vivo, and decreased biofilm formation and bacterial competitive advantage. In contrast, T6SS2-associated mutants presented a significant decrease only in the adherence to and invasion of mouse brain microvascular endothelial cell (BMEC) line bEnd.3 and brain tissue of the duck infection model. These results suggested that T6SS1 was involved in the proliferation of APEC in systemic infection, whereas VgrG-T6SS2 was responsible only for cerebral infection. Further study demonstrated that VgrG-T6SS2 was able to bind to the surface of bEnd.3 cells, whereas it did not bind to DF-1 (chicken embryo fibroblast) cells, which further proved the interaction of VgrG-T6SS2 with the surface of BMECs.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




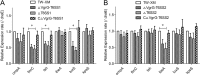

References
-
- Dho-Moulin M, Fairbrother JM. 1999. Avian pathogenic Escherichia coli (APEC). Vet. Res. 30:299–316 - PubMed
-
- Zhao L, Gao S, Huan H, Xu X, Zhu X, Yang W, Gao Q, Liu X. 2009. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E. coli in a murine urinary tract infection model and a chicken challenge model. Microbiology 155:1634–1644. 10.1099/mic.0.024869-0 - DOI - PubMed
-
- Gunther NT, Snyder JA, Lockatell V, Blomfield I, Johnson DE, Mobley HL. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 70:3344–3354. 10.1128/IAI.70.7.3344-3354.2002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

