Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole
- PMID: 24982133
- PMCID: PMC4104846
- DOI: 10.1073/pnas.1401777111
Cep164 triggers ciliogenesis by recruiting Tau tubulin kinase 2 to the mother centriole
Abstract
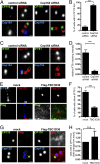
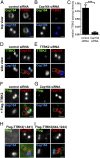
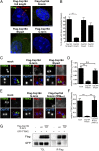
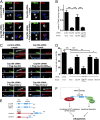
Primary cilia play critical roles in development and disease. Their assembly is triggered by mature centrioles (basal bodies) and requires centrosomal protein 164kDa (Cep164), a component of distal appendages. Here we show that loss of Cep164 leads to early defects in ciliogenesis, reminiscent of the phenotypic consequences of mutations in TTBK2 (Tau tubulin kinase 2). We identify Cep164 as a likely physiological substrate of TTBK2 and demonstrate that Cep164 and TTBK2 form a complex. We map the interaction domains and demonstrate that complex formation is crucial for the recruitment of TTBK2 to basal bodies. Remarkably, ciliogenesis can be restored in Cep164-depleted cells by expression of chimeric proteins in which TTBK2 is fused to the C-terminal centriole-targeting domain of Cep164. These findings indicate that one of the major functions of Cep164 in ciliogenesis is to recruit active TTBK2 to centrioles. Once positioned, TTBK2 then triggers key events required for ciliogenesis, including removal of CP110 and recruitment of intraflagellar transport proteins. In addition, our data suggest that TTBK2 also acts upstream of Cep164, contributing to the assembly of distal appendages.
Keywords: centrosome; primary cilium.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

