Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons
- PMID: 24982149
- PMCID: PMC4104922
- DOI: 10.1073/pnas.1403286111
Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons
Abstract
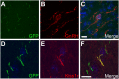
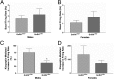
Most central neurons in the mammalian brain possess an appendage called a primary cilium that projects from the soma into the extracellular space. The importance of these organelles is highlighted by the fact that primary cilia dysfunction is associated with numerous neuropathologies, including hyperphagia-induced obesity, hypogonadism, and learning and memory deficits. Neuronal cilia are enriched for signaling molecules, including certain G protein-coupled receptors (GPCRs), suggesting that neuronal cilia sense and respond to neuromodulators in the extracellular space. However, the impact of cilia on signaling to central neurons has never been demonstrated. Here, we show that the kisspeptin receptor (Kiss1r), a GPCR that is activated by kisspeptin to regulate the onset of puberty and adult reproductive function, is enriched in cilia projecting from mouse gonadotropin-releasing hormone (GnRH) neurons. Interestingly, GnRH neurons in adult animals are multiciliated and the percentage of GnRH neurons possessing multiple Kiss1r-positive cilia increases during postnatal development in a progression that correlates with sexual maturation. Remarkably, disruption of cilia selectively on GnRH neurons leads to a significant reduction in kisspeptin-mediated GnRH neuronal activity. To our knowledge, this result is the first demonstration of cilia disruption affecting central neuronal activity and highlights the importance of cilia for proper GPCR signaling.
Keywords: GPR54; electrophysiology; neuronal primary cilia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ishikawa H, Marshall WF. Ciliogenesis: Building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12(4):222–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

