Genetic analysis of the Complexin trans-clamping model for cross-linking SNARE complexes in vivo
- PMID: 24982161
- PMCID: PMC4104896
- DOI: 10.1073/pnas.1409311111
Genetic analysis of the Complexin trans-clamping model for cross-linking SNARE complexes in vivo
Abstract
Complexin (Cpx) is a SNARE-binding protein that regulates neurotransmission by clamping spontaneous synaptic vesicle fusion in the absence of Ca(2+) influx while promoting evoked release in response to an action potential. Previous studies indicated Cpx may cross-link multiple SNARE complexes via a trans interaction to function as a fusion clamp. During Ca(2+) influx, Cpx is predicted to undergo a conformational switch and collapse onto a single SNARE complex in a cis-binding mode to activate vesicle release. To test this model in vivo, we performed structure-function studies of the Cpx protein in Drosophila. Using genetic rescue approaches with cpx mutants that disrupt SNARE cross-linking, we find that manipulations that are predicted to block formation of the trans SNARE array disrupt the clamping function of Cpx. Unexpectedly, these same mutants rescue action potential-triggered release, indicating trans-SNARE cross-linking by Cpx is not a prerequisite for triggering evoked fusion. In contrast, mutations that impair Cpx-mediated cis-SNARE interactions that are necessary for transition from an open to closed conformation fail to rescue evoked release defects in cpx mutants, although they clamp spontaneous release normally. Our in vivo genetic manipulations support several predictions made by the Cpx cross-linking model, but unexpected results suggest additional mechanisms are likely to exist that regulate Cpx's effects on SNARE-mediated fusion. Our findings also indicate that the inhibitory and activating functions of Cpx are genetically separable, and can be mapped to distinct molecular mechanisms that differentially regulate the SNARE fusion machinery.
Keywords: exocytosis; neurotransmitter release; synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

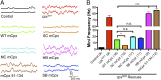
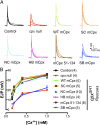

References
-
- Söllner T, et al. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362(6418):318–324. - PubMed
-
- Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92(6):759–772. - PubMed
-
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395(6700):347–353. - PubMed
-
- Li F, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14(10):890–896. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

