Engineered nanomedicine for myeloma and bone microenvironment targeting
- PMID: 24982170
- PMCID: PMC4104924
- DOI: 10.1073/pnas.1401337111
Engineered nanomedicine for myeloma and bone microenvironment targeting
Abstract
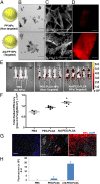
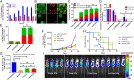
Bone is a favorable microenvironment for tumor growth and a frequent destination for metastatic cancer cells. Targeting cancers within the bone marrow remains a crucial oncologic challenge due to issues of drug availability and microenvironment-induced resistance. Herein, we engineered bone-homing polymeric nanoparticles (NPs) for spatiotemporally controlled delivery of therapeutics to bone, which diminish off-target effects and increase local drug concentrations. The NPs consist of poly(D,L-lactic-co-glycolic acid) (PLGA), polyethylene glycol (PEG), and bisphosphonate (or alendronate, a targeting ligand). The engineered NPs were formulated by blending varying ratios of the synthesized polymers: PLGA-b-PEG and alendronate-conjugated polymer PLGA-b-PEG-Ald, which ensured long circulation and targeting capabilities, respectively. The bone-binding ability of Ald-PEG-PLGA NPs was investigated by hydroxyapatite binding assays and ex vivo imaging of adherence to bone fragments. In vivo biodistribution of fluorescently labeled NPs showed higher retention, accumulation, and bone homing of targeted Ald-PEG-PLGA NPs, compared with nontargeted PEG-PLGA NPs. A library of bortezomib-loaded NPs (bone-targeted Ald-Bort-NPs and nontargeted Bort-NPs) were developed and screened for optimal physiochemical properties, drug loading, and release profiles. Ald-Bort-NPs were tested for efficacy in mouse models of multiple myeloma (MM). Results demonstrated significantly enhanced survival and decreased tumor burden in mice pretreated with Ald-Bort-NPs versus Ald-Empty-NPs (no drug) or the free drug. We also observed that bortezomib, as a pretreatment regimen, modified the bone microenvironment and enhanced bone strength and volume. Our findings suggest that NP-based anticancer therapies with bone-targeting specificity comprise a clinically relevant method of drug delivery that can inhibit tumor progression in MM.
Keywords: alendronate-PLGA-PEG; bisphosphonate; bone metastasis; targeting nanomedicine.
Conflict of interest statement
Conflict of interest statement: I.M.G. discloses her Advisory Board Membership with Novartis, Onyx, and BMS. O.C.F. discloses his financial interest in BIND Therapeutics, Selecta Biosciences, and Blend Therapeutics, three biotechnology companies developing nanoparticle technologies for medical applications. BIND, Selecta, and Blend did not support the aforementioned research, and currently these companies have no rights to any technology or intellectual property developed as part of this research.
Figures



References
-
- Roodman GD. Pathogenesis of myeloma bone disease. Leukemia. 2009;23(3):435–441. - PubMed
-
- Coleman RE. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. - PubMed
-
- Ozaki S, et al. Therapy with bortezomib plus dexamethasone induces osteoblast activation in responsive patients with multiple myeloma. Int J Hematol. 2007;86(2):180–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

