Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling
- PMID: 24982174
- PMCID: PMC4104912
- DOI: 10.1073/pnas.1405388111
Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling
Abstract
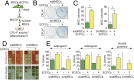
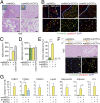
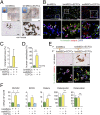
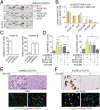
Endothelial colony-forming cells (ECFCs) are endothelial precursors that circulate in peripheral blood. Studies have demonstrated that human ECFCs have robust vasculogenic properties. However, whether ECFCs can exert trophic functions in support of specific stem cells in vivo remains largely unknown. Here, we sought to determine whether human ECFCs can function as paracrine mediators before the establishment of blood perfusion. We used two xenograft models of human mesenchymal stem cell (MSC) transplantation and studied how the presence of ECFCs modulates MSC engraftment and regenerative capacity in vivo. Human MSCs were isolated from white adipose tissue and bone marrow aspirates and were s.c. implanted into immunodeficient mice in the presence or absence of cord blood-derived ECFCs. MSC engraftment was regulated by ECFC-derived paracrine factors via platelet-derived growth factor BB (PDGF-BB)/platelet-derived growth factor receptor (PDGFR)-β signaling. Cotransplanting ECFCs significantly enhanced MSC engraftment by reducing early apoptosis and preserving stemness-related properties of PDGFR-β(+) MSCs, including the ability to repopulate secondary grafts. MSC engraftment was negligible in the absence of ECFCs and completely impaired in the presence of Tyrphostin AG1296, an inhibitor of PDGFR kinase. Additionally, transplanted MSCs displayed fate-restricted potential in vivo, with adipose tissue-derived and bone marrow-derived MSCs contributing exclusive differentiation along adipogenic and osteogenic lineages, respectively. This work demonstrates that blood-derived ECFCs can serve as paracrine mediators and regulate the regenerative potential of MSCs via PDGF-BB/PDGFR-β signaling. Our data suggest the systematic use of ECFCs as a means to improve MSC transplantation.
Keywords: adipogenesis; angiocrine factors; osteogenesis; vasculogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Ingram DA, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–2760. - PubMed
-
- Melero-Martin JM, et al. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109(11):4761–4768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

