Evolutionary origin of insect-Wolbachia nutritional mutualism
- PMID: 24982177
- PMCID: PMC4104916
- DOI: 10.1073/pnas.1409284111
Evolutionary origin of insect-Wolbachia nutritional mutualism
Abstract
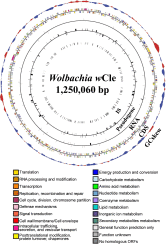
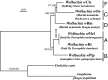
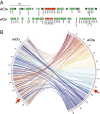
Obligate insect-bacterium nutritional mutualism is among the most sophisticated forms of symbiosis, wherein the host and the symbiont are integrated into a coherent biological entity and unable to survive without the partnership. Originally, however, such obligate symbiotic bacteria must have been derived from free-living bacteria. How highly specialized obligate mutualisms have arisen from less specialized associations is of interest. Here we address this evolutionary issue by focusing on an exceptional insect-Wolbachia nutritional mutualism. Although Wolbachia endosymbionts are ubiquitously found in diverse insects and generally regarded as facultative/parasitic associates for their insect hosts, a Wolbachia strain associated with the bedbug Cimex lectularius, designated as wCle, was shown to be essential for host's growth and reproduction via provisioning of B vitamins. We determined the 1,250,060-bp genome of wCle, which was generally similar to the genomes of insect-associated facultative Wolbachia strains, except for the presence of an operon encoding the complete biotin synthetic pathway that was acquired via lateral gene transfer presumably from a coinfecting endosymbiont Cardinium or Rickettsia. Nutritional and physiological experiments, in which wCle-infected and wCle-cured bedbugs of the same genetic background were fed on B-vitamin-manipulated blood meals via an artificial feeding system, demonstrated that wCle certainly synthesizes biotin, and the wCle-provisioned biotin significantly contributes to the host fitness. These findings strongly suggest that acquisition of a single gene cluster consisting of biotin synthesis genes underlies the bedbug-Wolbachia nutritional mutualism, uncovering an evolutionary transition from facultative symbiosis to obligate mutualism facilitated by lateral gene transfer in an endosymbiont lineage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Douglas AE. The Symbiotic Habit. Princeton: Princeton Univ Press; 2010. p. 202.
-
- Bourtzis K, Miller TA. Insect Symbiosis. Boca Raton, FL: CRC Press; 2003. p. 347.
-
- Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature. 2000;407(6800):81–86. - PubMed
-
- Akman L, et al. Genome sequence of the endocellular obligate symbiont of tsetse flies, Wigglesworthia glossinidia. Nat Genet. 2002;32(3):402–407. - PubMed
-
- Wernegreen JJ. Genome evolution in bacterial endosymbionts of insects. Nat Rev Genet. 2002;3(11):850–861. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

